Brain/Nerve Tumor Cells
- Background
- Applications
- Scientific Data
- FAQ
The human brain, the command center of the nervous system, is a complex organ composed of various types of cells, including nerve cells (neurons) and supporting cells (neuroglia). Brain and nerve tumor cells are a unique class of cancer cells that originate from the various cell types found in the central and peripheral nervous systems. These include glioma cells, which are derived from glial cells, and neuroblastoma cells, which arise from neural crest cells. These tumor cells exhibit distinct morphological, genetic, and behavioral characteristics that set them apart from other types of cancer cells.
Types of Brain/Nerve Tumor Cells
- Glioma cells. They are the most common type of brain tumor cells, originating from glial cells in the central nervous system. These include astrocytoma cells, oligodendroglioma cells, and ependymoma cells, among others. Glioma cells exhibit a wide range of malignancy grades, from low-grade (grade I-II) to highly aggressive, high-grade (grade III-IV) glioblastoma multiforme (GBM) cells.
- Neuroblastoma cells. They are derived from neural crest cells and are the most common extracranial solid tumors in children. These cells are found in the sympathetic nervous system and are known for their aggressive and heterogeneous nature. Neuroblastoma cells can exhibit varying degrees of differentiation, from highly undifferentiated and aggressive forms to more differentiated and less aggressive subtypes.
- Meningioma cells. They originate from the meningeal cells that line the outer covering of the brain and spinal cord. While often considered benign, some meningioma subtypes can exhibit more aggressive behavior and are associated with a higher risk of recurrence.
- Schwannoma cells. They are derived from Schwann cells, which are responsible for the myelination of peripheral nerves. These tumor cells are commonly associated with conditions like neurofibromatosis and can lead to the development of benign or malignant peripheral nerve sheath tumors.
- Pituitary adenoma cells. They originate from the anterior pituitary gland and can lead to the development of pituitary adenomas, which are often benign but can still cause significant hormonal imbalances and neurological complications.
Drug discovery and screening
One of the primary applications of brain and nerve tumor cells is in the field of drug discovery and screening. These specialized cells provide an excellent in vitro model for testing the efficacy and safety of potential therapeutic compounds targeting brain and nervous system cancers. Exposing brain and nerve tumor cells to various drugs or drug candidates, researchers can gain crucial insights into their mechanisms of action, identify potential targets, and evaluate the susceptibility of different tumor subtypes to specific treatments.
Genetic and molecular profiling
Brain and nerve tumor cells are also extensively used in genetic and molecular profiling studies. Analyzing the unique genomic, epigenomic, and transcriptomic profiles of these cells, researchers can uncover the underlying genetic alterations and signaling pathways that drive the development and progression of brain and nervous system cancers. This knowledge is essential for the development of personalized treatment strategies and the identification of novel therapeutic targets.
Tumor heterogeneity and microenvironment
Another important aspect of brain and nerve tumor cell research is the exploration of tumor heterogeneity and the tumor microenvironment. These specialized cells often exhibit significant intra- and inter-tumoral heterogeneity, reflecting the complex and dynamic nature of brain and nervous system cancers. By studying the interactions between brain and nerve tumor cells and their surrounding stromal components, such as immune cells and the extracellular matrix, researchers can gain a deeper understanding of the factors that influence tumor growth, invasion, and response to treatment.
Preferential Glucose Uptake by BTICs Under Restricted Nutrition
Like all cancers, brain tumors require a continuous source of energy and molecular resources for new cell production. De novo synthesis of molecular building blocks for cell growth requires glucose for energy production. Highly efficient glucose uptake could therefore provide a competitive advantage contributing to tumor maintenance, particularly with restricted glucose levels.
A glucose oxidase colorimetric assay after a pulse post nutrient starvation to measure glucose in cell lysates demonstrated brain tumor-initiating cells (BTICs) had higher levels of glucose than matched non-BTICs (Fig. 1a). To directly compare the uptake of glucose in GBM tumor cell subsets, a fluorescent glucose analog, 2-[N-(7-nitrobenz-2-oxa-1,3-diaxol-4-yl)amino]-2-deoxyglucose (2-NBDG) that mimics glucose19 was used. Bulk GBM cells were incubated with 2-NBDG and an antibody against the BTIC marker CD133. Flow cytometry demonstrated that CD133 expressing cells consistently displayed higher 2-NBDG fluorescence (Fig. 1b). Ex vivo multiphoton imaging of labeled GBM subpopulations in brain slice cultures confirmed that BTICs outcompete for glucose with surrounding brain and differentiated tumor cells (Fig. 1c-g). Quantification of fluorescence visualized through multiphoton imaging with Imaris reconstruction20 demonstrated that the uptake of NBDG was greater than two-fold higher in the BTICs, consistent with results in the flow cytometry assays. Together, these data indicate that BTICs preferentially uptake glucose relative to their non-BTIC counterparts.
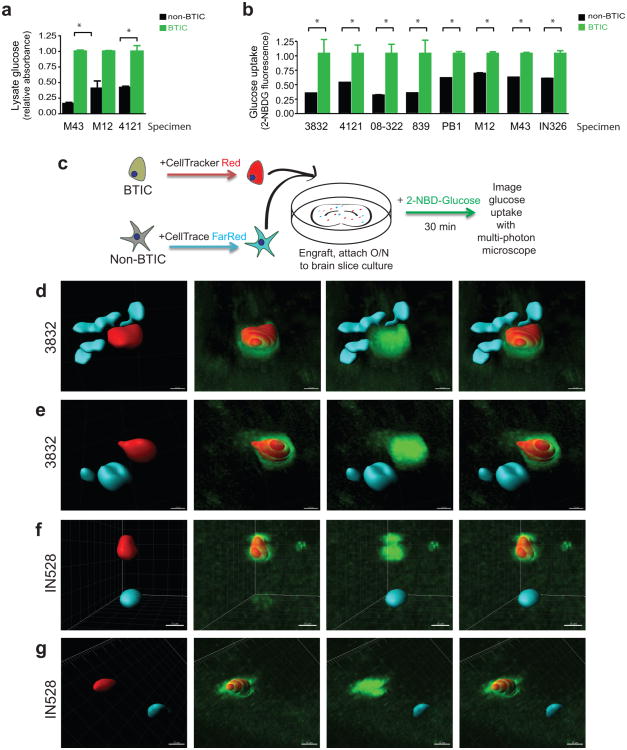 Fig.
1 BTICs preferentially uptake glucose. (Flavahan WA, et al, 2013)
Fig.
1 BTICs preferentially uptake glucose. (Flavahan WA, et al, 2013)
PRMT5 Inhibition Induces Aberrant Alternative Splicing in Glioblastoma
Glioblastoma (GBM) is a deadly cancer in which cancer stem cells (CSCs) sustain tumor growth and contribute to therapeutic resistance. Protein arginine methyltransferase 5 (PRMT5) has recently emerged as a promising target in GBM.
To better understand the cellular mechanisms that may account for the attenuation of growth observed upon PRMT5 inhibition in GSCs, bulk RNA-seq was performed on three GSC lines (G561, G564, and G583) after a 3-day treatment with GSK591 or the inactive control, SGC2096, and analyzed the differential effect on gene expression. 646 genes were identified that were significantly differentially expressed between the two treatments (false discovery rate (FDR)<0.05) (Fig. 2a). Gene set enrichment analysis (GSEA) of the differentially expressed genes (DEGs) revealed enrichment in genes involved in spliceosome complex-related pathways, corroborating previous reports that PRMT5 inhibition has a profound effect on the maintenance of splicing fidelity through disruption of arginine methylation of splicing factors and RNA-binding proteins.
RNA-seq data from the above three GSC lines were analyzed to identify disruptive alternative splicing events (ASEs). A total of 11,582 statistically significant differentially spliced events were identified in the three GSC lines upon PRMT5 inhibition. Only a fraction of these events (3%, 317 events) were common in all three GSC lines, suggesting that, although PRMT5 inhibition leads to widespread splicing disruption in the GSC lines, the alternatively spliced transcripts varied widely from sample to sample (Fig. 2c). The 317 common ASEs (occurring in 274 genes) included cassette exons (CEs), mutually exclusive exons (MXEs), alternative splicing at the 3' or 5' site (A3/5SSs), and retained introns (RIs), with the highest number of ASEs comprising CEs and Ris. Analysis of the predicted protein impact of the ASEs by VASTdb revealed that 69% of ASEs had predicted disruptive effects on the proteins they encode, with CEs and RIs resulting in the most deleterious impact on their targets (Fig. 2d). Relative to the null expectation whereby each ASE should be equally likely to be included or excluded after treatment, independently of its predicted effect, ASEs observed after PRMT5 inhibition were significantly more often predicted to cause open reading frame (ORF) disruption.
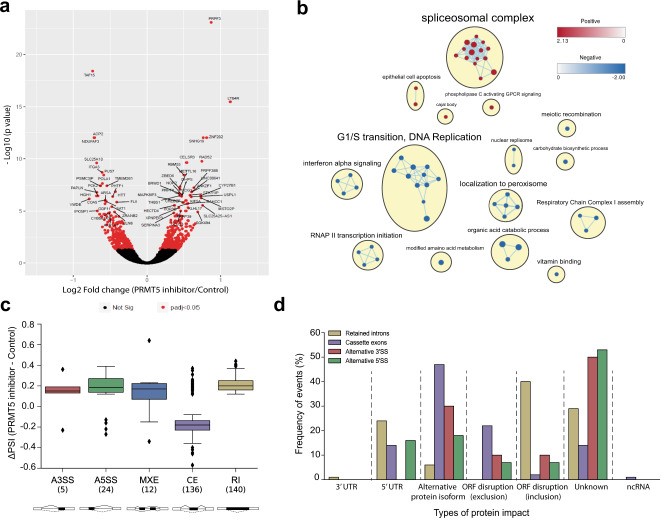 Fig.
2 PRMT5 inhibition leads to the deregulation of alternative splicing. (Sachamitr P, et al., 2021)
Fig.
2 PRMT5 inhibition leads to the deregulation of alternative splicing. (Sachamitr P, et al., 2021)
Brain and nerve tumor cells exhibit genetic and molecular alterations that lead to uncontrolled cell growth, increased proliferation, and invasion of surrounding tissues. They often have impaired mechanisms for regulating cell division and survival, setting them apart from their healthy counterparts.
Well-characterized brain and nerve tumor cell lines, such as those provided by Creative Bioarray, offer several advantages for researchers, including the availability of a consistent and reliable source of cells, the ability to conduct reproducible experiments, and the opportunity to study specific subtypes of brain and nerve tumors in a controlled and standardized manner.
Studying brain and nerve tumor cells can be challenging due to their inherent genetic and cellular heterogeneity, as well as the complex tumor microenvironment that influences their behavior. Additionally, the location and accessibility of brain and nervous system tumors can pose practical difficulties for researchers.
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: Species: human, Caucasian; Tumor: neuroblastoma
Description: Species: human male;Tumor: neuroblastoma;Derived from: bone marrow metastasis
Description: Species: human; Tissue: brain; Tumor: glioblastoma; Transfected with binase (ATP binding site 721), ...
Description: Species: human; Tissue: brain; Tumor: glioblastoma; Transfected with: Transfected with wt EGFR
Description: Established from a neuroblastoma metastasis at an adrenal site of a 4-year-old white boy in 1986; ...
Description: Established from the biopsy specimens from a 42-year-old woman with glioma (histologically defined ...
Description: Established from the glioblastoma multiforme (right parietal, at diagnosis) of a 67-year-old woman ...
Description: Established from a large retroperitoneal mass excised from a 14-month-old girl with neuroblastoma ...
Description: Established from the malignant brain tumor of a 49-year-old man with glioblastoma in 1995
Description: Established from the temporal lobe brain biopsy from a 63-year-old man with glioblastoma multiforme ...
Description: Established from the frontal brain lobe of a 54-year-old woman with glioblastoma multiforme in 1989 ...
Description: Established in 1973 from an autopsy specimen of a 18-month-old boy with stage IV neuroblastoma ...
Description: Established in 1977 from a bone marrow metastasis of a 2-year-old boy with stage IV neuroblastoma
Description: Established from the primary adrenal tumor resected prior to treatment from a 3-year-old boy with ...
Description: Established in 1974 from a bone marrow metastasis of a 10-month-old girl with neuroblastoma; cells ...
Description: Established from the primary tumor of a 3-year-old girl with neuroblastoma (clinical Stage IV)
Description: Established from a bone marrow metastasis of a 6-year-old boy with neuroblastoma (clinical Stage IV)
Description: Established from a stage III abdominal tumor of a 16-month-old girl with neuroblastoma; cells were ...