MHH-NB-11
Cat.No.: CSC-C0304
Species: Homo sapiens (Human)
Source: Adrenal Gland Metastasis
Morphology: adherent, fibroblast-like cells growing as monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin -, cytokeratin-7 -, cytokeratin-8 -, cytokeratin-17 -, cytokeratin-18 -, desmin -, endothel -, GFAP -, neurofilament +, vimentin +
Viruses: ELISA: reve
MHH-NB-11 is a human neuroblastoma cell line established from a bone marrow metastasis of a 2-year-old female with stage IV MYCN-amplified neuroblastoma. MHH-NB-11 is an aggressive pediatric cancer derived from the sympathoadrenal lineage of the neural crest. Morphologically, cells are small and round-to-polygonal with high nuclear to cytoplasmic ratio and grow semi-adherent in clusters or in suspension. Genetically, MHH-NB-11 is characterized by the presence of MYCN amplification, ALK mutations, TP53 mutations, and ATRX mutations which is a common and clinically relevant pattern of genomic alterations in high-risk neuroblastoma. Phenotypically, this cell line is highly tumorigenic, can metastasize to bone and adrenal tissues in xenograft models, and is intrinsically resistant to chemotherapies (cisplatin, etoposide) to model the unique characteristics of relapsed/refractory disease. Widely used in drug discovery (targeted therapies, CAR-T cells), mechanistic research (MYCN oncogenesis, metabolic reprogramming), and biomarker studies.
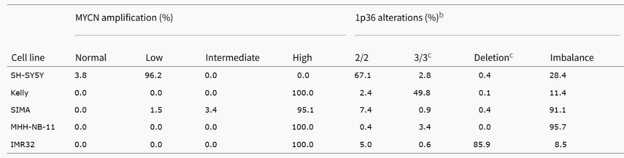
MYCN Amplification Status, 1p36 Alterations and Ploidy Level Determined by FISH Analysis in Neuroblastoma Cell Lines
Neuroblastoma is a common solid tumor in infants, with MYCN amplification and 1p36 deletion being associated with poor prognosis. The expression of MYCN and c‑myb declines during neuroblastoma cell differentiation, and E2F1 activates the MYCN promoter. However, the mechanisms behind MYCN overexpression and amplification are not fully understood. To explore the mechanisms of MYCN overexpression and amplification, this study used quantitative polymerase chain reaction to measure mRNA levels and MYCN gene copy number in five neuroblastoma cell lines.
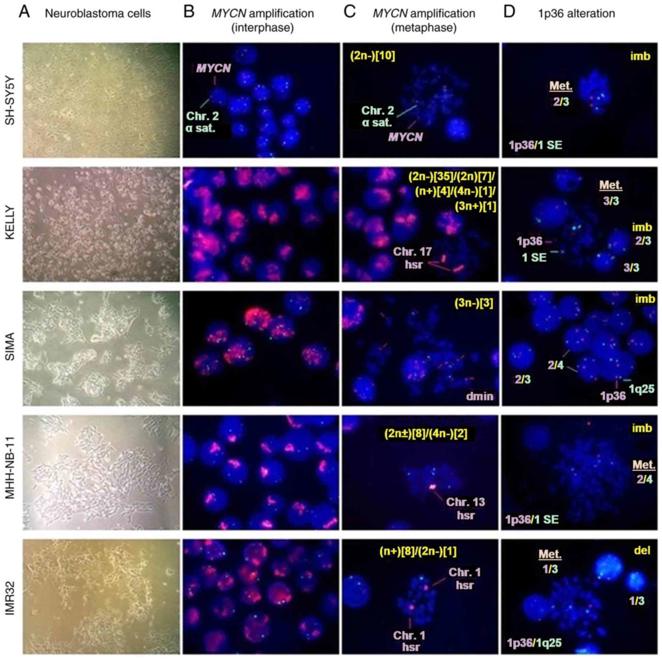
The FISH analyses demonstrated that MYCN amplification was high in Kelly, SIMA, MHH-NB-11, and IMR32 cells (Fig. 1), and a different fluorescence signal intensity was shown in Fig. 2B. In SH-SY5Y cells (non-MYCN-amplified cells), the most common number of MYCN copies was three (93.1%) (Fig. 1 and Fig. 2B). The FISH of metaphase spread also showed HSRs in Kelly, MHH-NB-11 and IMR32 cells, and double minutes in SIMA cells (Fig. 2C), respectively. Ploidy levels were also calculated (Fig. 2C). IMR32 had the highest deletion rate of 1p36 in this study, and others had a lower percentage (Fig. 1 and Fig. 2D). The percentage of cells with 1p36 imbalance in SIMA and MHH-NB-11 was significantly higher than in SH-SY5Y, Kelly, and IMR32 (Fig. 1 and Fig. 2D). Kelly had a higher percentage of 1p36-3/3 balanced alteration, which was negatively associated with 1p36 deletion (Fig. 1 and Fig. 2D). In conclusion, FISH analyses revealed that all neuroblastoma cell lines had aneuploid cell clones. The MYCN-amplified cell lines had MYCN amplification and at least one 1p36 alteration, whereas SH-SY5Y cells had 1p36 imbalance but no MYCN amplification.
In Vitro and In Vivo Efficacy of SHP099 in Reoccurring/High-Risk Neuroblastoma Tumors
Reoccurring/high-risk neuroblastoma (NB) tumors frequently have non-RAS/RAF mutations in the MAPK pathway suggesting that MEK/ERK activation is essential for the survival of these tumors. MEK inhibitors have had very limited efficacy and shown dose-limiting toxicities in preclinical screens. Here, Cai’s team present an alternative strategy to target the MAPK pathway in high-risk NB.
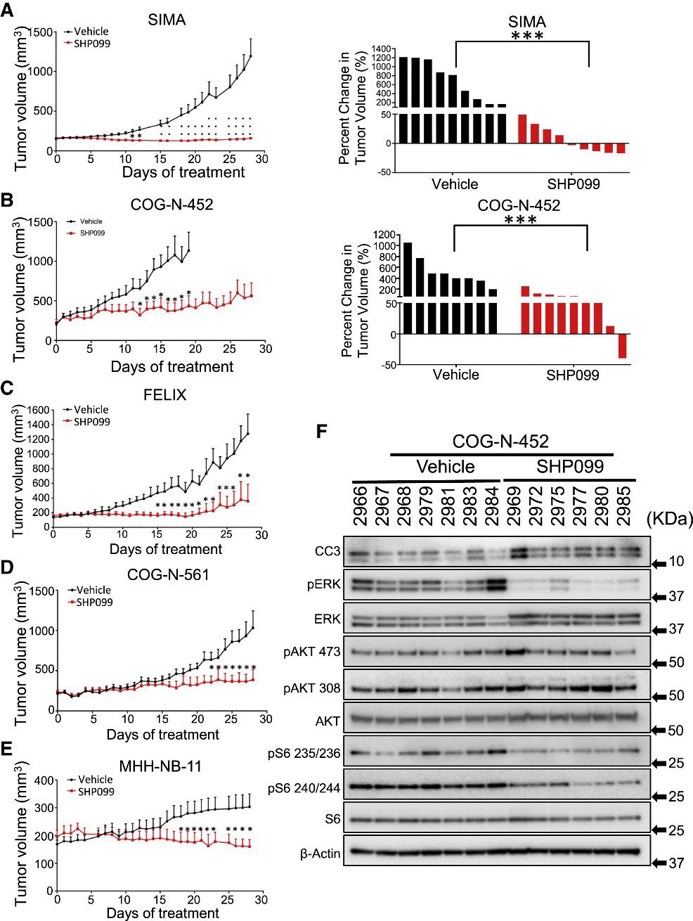
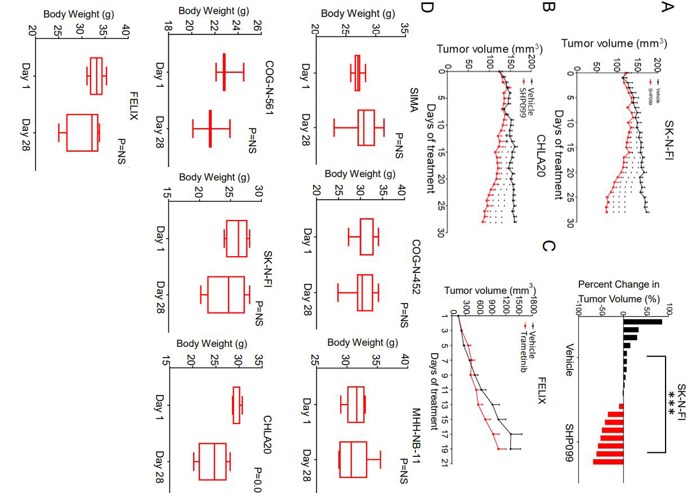
They assessed the activity of SHP099 in seven mouse models of high-risk NB (MYCN-amplified SIMA, MHH-NB-11, COG-N-452, MYCN-WT SK-N-FI and FELIX, and PDX models CHLA20, COG-N-452 and COG-N-561). Immunocompromised mice were used to create tumors before administering treatment with either SHP099 at 75 mg/kg/day or vehicle. The SIMA model showed complete growth inhibition during a 4-week period (Fig. 3A) and 5 out of 9 tumors shrank. In the COG-N-452 PDX, tumor growth was significantly slowed (Fig. 3B). SHP099 controlled tumor growth in FELIX, COG-N-561 (Fig. 3C and D), and MHH-NB-11 models (Fig. 3E). Tumors in the slower growing SK-N-FI and CHLA20 models shrank or decreased in volume with SHP099 treatment (Fig. 4). Assessment of treated tumors revealed on-target inhibition of pERK and evidence of cell death (cleaved caspase 3) (Fig. 3F). There was no feedback activation of mTORC2, and targets of mTORC1 were downregulated (Fig. 3F). Trametinib was inactive in vivo at an effective dose in BRAF mutant models (Fig. 4C). Most mice did not lose more than 10% of their body weight (Fig. 4D). Thus, the in vitro activity of SHP099 was effectively recapitulated in vivo.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells