Colorimetric Cell Viability Assay
The biochemical procedure is based on the activity of mitochondrial enzymes which are inactivated shortly after cell death. This method was found to be very efficient in assessing the viability of cells. A colorimetric method based on tetrazolium salts (e.g., MTT, XTT, CCK-8/WST-8) are especially useful for assaying the quantification of viable cells, because they are cleaved to form a formazan dye only by metabolic active cells. The formazan dye formed can be directly quantified using a scanning multi-well spectrophotometer, which enables on-line computer processing of the data (data collection, calculation and report generation) and, thereby, allows the rapid and convenient handling of a high number of samples.
MTT
The succinate dehydrogenase in the mitochondria of living cell can reduce exogenous MTT to water-insoluble purple crystalline formazan and deposit in the cells, whereas dead cells do not. Dimethyl sulfoxide (DMSO) is capable of dissolving the formazan in the cells, and its absorbance can be measured at 490 nm by an enzyme-linked immunosorbent assay, which indirectly reflects the number of viable cells. The method has been widely used for the detection of biological active factors, large-scale anti-tumor drug screening, cytotoxicity test, and tumor radiosensitivity measurement.
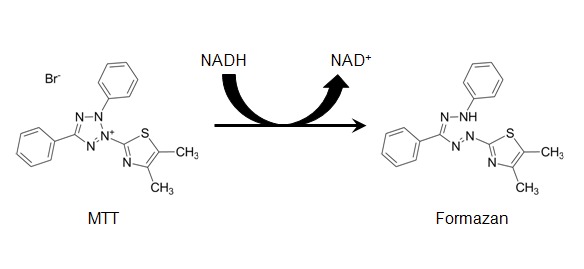 Figure 1. Chemical structures of yellow MTT and purple formazan product.
Figure 1. Chemical structures of yellow MTT and purple formazan product.
Advantages
- High sensitivity and economy.
Disadvantages
- The crystal formation products are insoluble and need to be dissolved before detection which increases the workload and affects the accuracy of the results.
- The organic solvents DMSO are harmful to the experimenter.
- The MTT reaction is time consuming.
XTT
Tetrazolium salt XTT has been developed for determining the number of viable cells in the medium. It is based on the reduction of XTT into a water-soluble orange yellow formazan compound by mitochondrial dehydrogenase. Since these enzymes are inactivated shortly after cell death, it is a reliable method for detecting viable cells. The absorbance of the formazan products can be measured directly from a 96-well plate without additional processing in combination with an electronic coupling agent such as PMS.
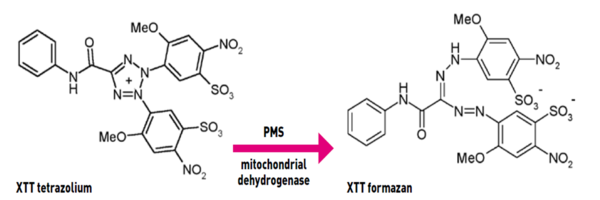 Figure 2. The reduction of XTT to form the colored formazan derivative.
Figure 2. The reduction of XTT to form the colored formazan derivative.
Advantages
- Easy to use, eliminating the need to wash cells.
- Quick detection.
- High sensitivity, and even can measure lower cell density.
- Repeatability is better than MTT.
Disadvantages
- The aqueous solutions of XTT are unstable and require cryopreservation or ready-to-use.
CCK-8/WST-8
CCK-8/WST-8 is reduced by the dehydrogenase in the cell mitochondria to the highly water-soluble yellow formazan product under the action of the electron carrier 1-methoxy-5-methylphenazine dimethyl sulfate (1-Methoxy PMS). The absorbance of the light is measured at 450 nm, which indirectly reflects the number of viable cells. The method has also been used for the detection of biological active factors, large-scale anti-tumor drug screening, and cytotoxicity test.
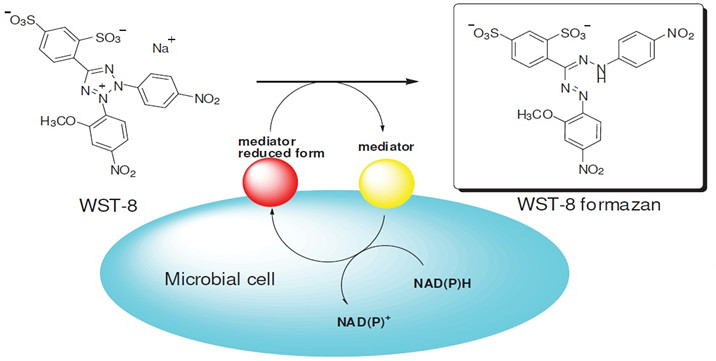 Figure 3. The cell viability detection mechanism of WST-8.
Figure 3. The cell viability detection mechanism of WST-8.
Advantages
- Easy to use, no radioisotopes and organic solvents.
- Quick detection and high sensitivity.
- Repeatability is better.
- Little cytotoxicity.
- No prefabrication.
Disadvantages
- More expensive compared with MTT.
- The color of the CCK-8 reagent is close to the color of the medium containing phenol red, which is prone to cause leakage or increase.
Table 1. The comparison of three methods
| MTT | XTT | CCK-8/WST-8 | |
| Water solubility of formazan | Water-insoluble | Water-soluble | Water-soluble |
| Detection wavelength | 490 nm | 450 nm | 450 nm |
| Traits | Powder | Solution | Solution |
| Instructions | Used as a solution | Ready-to-use | No prefabrication |
| Whether to use organic solvents | DMSO | - | - |
| Convenience | + | ++ | +++ |
| Repeatability | + | ++ | ++ |
| Stability | ++ | + | ++ |
Experimental Protocol Summary
- In general, seed cells at densities range from 5000 to 10,000 per well should reach an optimal population densities within 48-72 hours.
- For adherent cells, remove the medium and replace it with 100 µL of fresh medium. For suspension cells, centrifuge the plates and carefully remove as much medium as possible and replace it with 100 µL of fresh medium.
- Add tetrazolium salts stock solution according your instruction manual to each well.
- Incubate at 37°C, the incubation time can be shortened at high cell densities (>100,000 cells per well).
- Add 100 µL of the organic solvents to each well and mix thoroughly using the pipette (not required for XTT and CCK-8/WST-8).
- Return the plates to the humidified chamber for the optimized-assay incubation time.
- Shake the plate gently and measure the absorbance.
Cell Services:
Cell Line Testing and Assays: