Proliferating Cell Nuclear Antigen (PCNA) Assay
Proliferating cell nuclear antigen (PCNA) is a homotrimeric protein known as the cofactor of DNA polymerase δ, which is responsible for leading strand DNA replication. PCNA was originally identified as an antigen that is expressed in the nucleus during the DNA synthesis phase of the cell cycle. Levels of PCNA protein are low in quiescent cells and increase under the stimulation of mitogen with peak protein levels localized in the nucleus during S-phase. PCNA interacts with a lot of proteins involved in DNA replication, cell cycle control, and cell cycle checkpoint control.
The fact that PCNA expression is significantly elevated during the S and G2 phases of the cell cycle, but is very low in quiescent cells, makes this protein a good marker for cell proliferation assay. Since then, the quantitative measurement of PCNA in human cell and tissue extracts has been extensively used in cancer diagnosis and prognosis.
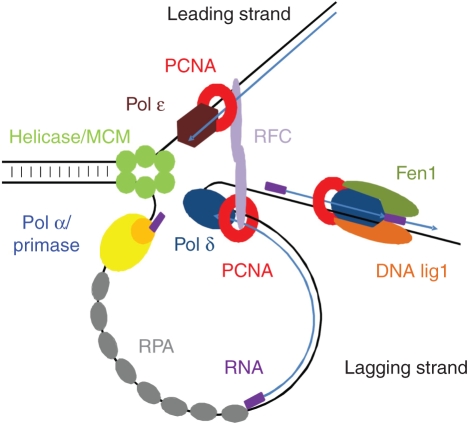 Figure 1. PCNA function in DNA replication.
Figure 1. PCNA function in DNA replication.
Materials
- Anti-PCNA antibody
- Secondary antibody
- PCNA lyophilized recombinant protein
- Antibody diluent
- Wash buffer
- Cell extraction buffer
- TMB substrate
- Stop solution
- Pre-coated 96-well microplate
- Deionized water
- Phenylmethylsulfonyl fluoride (PMSF)
- Microplate reader
- Multi- and single-channel pipettes
- Plate shaker
Standard Curve
Prepare serially diluted standards immediately prior to use. The following table describes the preparation of a standard curve.
Table 1. Preparation of PCNA standards by serial dilution.
| Volume of PCNA solution | Volume of diluent | Final PCNA concentration | |
| A | PCNA lyophilized protein sample (600ng) | 1 mL | 600 ng/mL |
| B | 25 μL solution A | 275 μL | 50 ng/mL |
| C | 150 μL solution B | 150 μL | 25 ng/mL |
| D | 150 μL solution C | 150 μL | 12.5 ng/mL |
| E | 150 μL solution D | 150 μL | 6.25 ng/mL |
| F | 150 μL solution E | 150 μL | 3.13 ng/mL |
| G | 150 μL solution F | 150 μL | 1.56 ng/mL |
| H | 150 μL solution G | 150 μL | 0.78 ng/mL |
| I | -- | 150 μL | 0 ng/mL |
Preparation of Extracts from Cells
- For suspension cells, collect by centrifugation.
- For adherent cells, directly add chilled cell extraction buffer or scrape the cells into microfuge tubes.
- Centrifuge at 500 g for 5 minutes at 4ºC.
- Wash twice with PBS.
- Add chilled cell extraction buffer and incubate for 20 minutes on ice.
- Centrifuge at 18,000 g for 20 minutes at 4°C.
- Transfer the supernatants to clean tubes and discard the pellets.
Preparation of Extracts from Tissue Homogenates
- Tissue lysates are typically prepared by homogenization that is first minced and thoroughly rinsed in PBS to remove blood.
- Homogenize 100-200 mg of the wet tissue in 500 µL-1 mL of chilled cell extraction buffer. For lower amounts of tissue, adjust the volumes accordingly.
- Incubate on ice for 20 minutes.
- Centrifuge at 18,000 g for 20 minutes at 4°C.
- Transfer the supernatants to clean tubes and discard the pellets.
Assay Procedure
- Add 50 µL of all samples or standards to the pre-coated 96-well plate.
- Incubate at 37°C for at least 2 hours or 4°C overnight.
- Wash each well 3 times with 250 µL wash buffer. Complete removal of liquid at each step is essential for good performance. After the last wash, invert the plate and tap it against clean paper towels to remove excess wash buffer.
- Add 50 µL of Anti-PCNA antibody to each well and incubate at 37°C for 1 hour on a plate shaker.
- Wash each well 3 times with 250 µL wash buffer. Complete removal of liquid at each step. After the last wash, invert the plate and tap it against clean paper towels to remove excess wash buffer.
- Add 50 µL of secondary antibody to each well and incubate at 37°C for 1 hour on a plate shaker.
- Wash each well 3 times with 250 µL wash buffer. Completely remove the liquid at each step. After the last wash, invert the plate and tap it against clean paper towels to remove excess wash buffer.
- Add 100 µL of TMB substrate to each well and incubate for 10 minutes on a plate shaker.
Note: Watch plate carefully, if the color changes rapidly, the reaction may need to be stopped sooner to prevent saturation. - Add 100 µL of stop solution to each well. Shake the plate for 1 minute to mix.
Note: Results should be read immediately as the color will fade over time. - Record the absorbance of each well at 450 nm.
Notes:
- Sample values higher than the highest standard should be further diluted in the appropriate sample dilution buffers.
- Avoid foaming or bubbles when mixing or reconstituting components.
- Avoid cross contamination of samples or reagents by changing the tips between sample, standard and reagent additions.
- Ensure the plate is properly sealed or covered during incubation steps.
- Completely remove all solutions and buffers during wash steps to minimize the background.
- All samples should be mixed thoroughly and gently.
- Avoid multiple freeze/thaw of samples.
- Incubate plate on a plate shaker in all steps of incubation.
- When generating positive control samples, it is recommended to change the pipette tips after each step.
References
- Connolly K. M. et al.; Evaluation of proliferating cell nuclear antigen (PCNA) as an endogenous marker of cell proliferation in rat liver: a dual-stain comparison with 5-bromo-2’-deoxyuridine. The Journal of Histochemistry and Cytochemistry, 1993, 41(1): 1-6.
- Wojciech Strzalka, et al.; Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot, 2011, 107(7): 1127-1140.
- Jeroen Essers, et al.; Nuclear dynamics of PCNA in DNA replication and repair. Molecular and Cellular Biology, 2005, 25(21): 9350-935.
Cell Services:
Cell Line Testing and Assays: