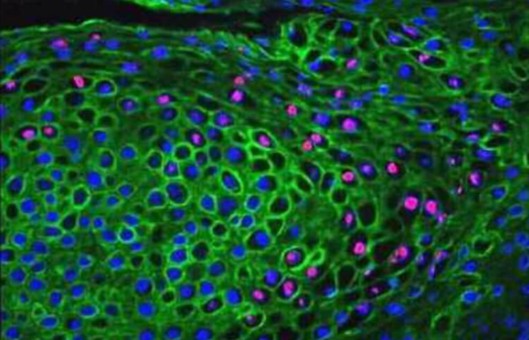
Whole-Mount Fluorescent IHC Protocol
GUIDELINE
The method provides a protocol for whole-mount fluorescent immunohistochemistry (IHC). The advantage of using fluorescence to stain whole mount sections is that confocal microscopy can be used to section through the larger embryo or tissue sample without having to manually section onto slides. This gives a clearer idea of where the target protein of interest is expressed within the tissues.
METHODS
- Place the embryo in 5 ml bijous in 4% paraformaldehyde. Leave to fix 4°C. The time required will need optimization. We suggest trying between 2 hours and overnight.
- Wash 3 times in PBS 0.5-1% Triton 30 min each time.
- Incubate the embryos twice for 1 hr in a block (PBS 1% Triton 10% FCS, 0.2% sodium azide), at room temperature.
- Wash embryos 2 times in blocking buffer.
- Transfer embryos using a Pasteur pipette with the end cut off to a 2 ml tube. Add primary antibody at the required dilution/concentration.
- It is recommended that as incubations can be very long in whole-mount staining, the antibody should be diluted in a blocking buffer containing 0.02% sodium azide to prevent microbial growth.
- Incubate for 1 to 4 days on a gentle rotation device at 4°C.
- This incubation time will require some optimization depending on the antibody and also the size of the embryo.
- Wash embryos 3 times 1 hr in PBS 1% Triton 10% FCS.
- Wash 3 times 10 minutes in PBS 1% Triton.
- Wash embryos 3 times 1 hr in PBS 1% Triton 10% FCS 0.2% sodium azide.
- Wash 3 times 10 minutes in PBS 1% Triton.
- Add secondary antibody in blocking buffer (PBS 1% Triton 10% FCS 0.2% sodium azide).
- Incubate for 2 to 4 days with gentle rotation at 4°C.
- Wash 3 times 10 minutes in PBS 1% triton.
- Mount and view embryos. Store at 4°C in the dark until analysis.
Creative Bioarray Relevant Recommendations
- Creative Bioarray offers a comprehensive IHC service from project design, and marker selection to image completion and data analysis. We are dedicated to satisfying every customer and assisting them to achieve their specific research goals.
- We also provide a range of normal and diseased tissue blocks. Our high-quality tissues are banked under rigorous collection protocols to ensure that both paraffin-embedded and frozen tissues are all well-characterized, clinically annotated tissues needed for critical experiments.
View our full range of tissue blocks and find what you need!
NOTES
- Place the sample in 100% glycerol for 48 hours. When the sample is fully equilibrated with the glycerol (i.e., it is fully perfused with the glycerol) it will sink to the bottom of the vial. Ensure the sample is at this stage before proceeding.
- 75% glycerol has approximately the same density as gelatin which is used to mount and set the samples on a slide. Therefore, samples should be equilibrated in 75% glycerol after staining for approximately 15 minutes (again, when equilibrated, the sample should sink).
- Place in 50% glycerol until the sample sinks. The embryo can be imaged at this stage, or mounted whole in the glycerol on a slide. Use grease around the edges of the coverslip for protection.
- If the sample is to be embedded in gelatin and sectioned on a vibratome; place 20% gelatin pre-warmed to 65oC. Leave for approximately 30 minutes to equilibrate before taking out the sample to mount. When equilibrated in the gelatin, the sample should sink to the bottom.