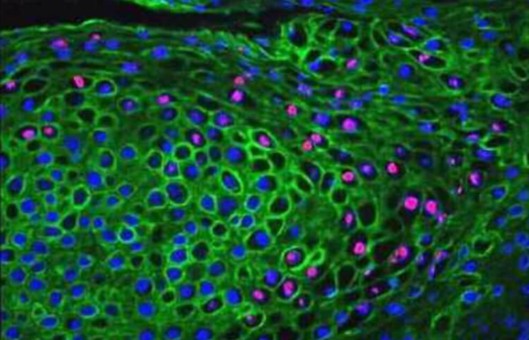
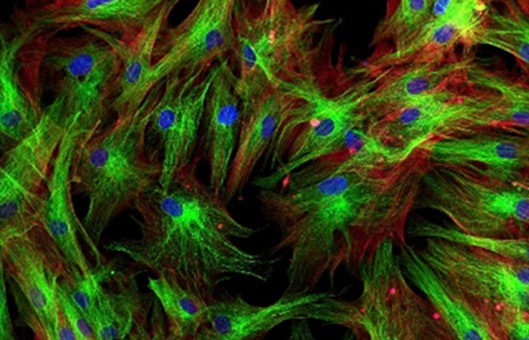
Simultaneous Double IF Protocol
GUIDELINE
In order to be able to examine the co-distribution of two (or more) different antigens in the same sample, a double immunofluorescence procedure can be carried out. Primary antibodies raised in different species can be used either in parallel (in a mixture) or in a sequential way. We provide a protocol for immunofluorescent double staining incubating the antibodies together.
METHODS
Preparation of Slides and Samples
- Cell lines, cytology smears, cytospin preparations. Coat coverslips with polyethylineimine or poly-L-lysine for 1 hr at room temperature. Rinse coverslips well with sterile H2O (3 times 5 min each). Allow coverslips to dry completely and sterilize them under UV light for at least 4 hr. Grow cells on glass coverslips or prepare cytospin or smear preparation. Rinse briefly in phosphate-buffered saline (PBS).
- Frozen (cryostat) sections. Snap frozen fresh tissue in liquid nitrogen or isopentane pre-cooled in liquid nitrogen. Store frozen blocks at -80°C. Cut 4-8 μm thick cryostat sections and mount on super frost or gelatin coated slides. You can store slides at -80°C until needed. Before IF staining, warm up slides at room temperature for 30 minutes.
- Paraffin-embedded sections. Deparaffinize sections in xylene 2 x 5 min. Hydrate with 100% ethanol 2 x 3 min. Hydrate with 95% ethanol 1 min. Rinse in distilled water and then follow procedure for fixation and antigen retrieval as required.
Fixation
- Fix the samples either in ice-cold methanol, acetone (1-10 min) or in 3-4% paraformaldehyde in PBS pH 7.4 for 15 min at room temperature.
- Wash the samples twice with ice cold PBS.
Permeabilization
- Incubate the samples for 10 min with PBS containing 0.25% Triton X-100 (or 100 μM digitonin or 0.5% saponin). Triton X-100 is the most popular detergent for improving the penetration of the antibody. However, it is not appropriate for the use of membrane-associated antigens since it destroys membranes.
- Wash cells in PBS three times for 5 min.
Blocking and Simultaneous Incubation
- Incubate cells with 1% BSA in PBST for 30 min to block unspecific binding of the antibodies.
- Incubate cells in the mixture of two primary antibodies (e.g. rabbit against human target-1 and mouse against human target-2, if the targets are human proteins) in 1% BSA in PBST in a humidified chamber for 1 hr at room temperature or overnight at 4°C.
- Decant the mixture solution and wash the cells three times in PBS, 5 min each wash.
- Incubate cells with the mixture of two secondary antibodies which are raised in different species (with two different fluorochromes, i.e. Texas Red-conjugated against rabbit and FITC-conjugated against mouse) in 1% BSA for 1 hr at room temperature in dark.
- Decant the mixture of the secondary antibody solution and wash three times with PBS for 5 min each in dark.
Counter Staining
- Incubate cells on 0.1-1 μg/ml Hoechst or DAPI (DNA stain) for 1 min.
- Rinse with PBS.
Mounting
- Mount coverslip with a drop of mounting medium.
- Seal coverslip with nail polish to prevent drying and movement under microscope.
- Store in dark at -20°C or 4°C.
NOTES
If the target protein is expressed intracellularly, it is very important to permeabilize the cells. Acetone fixed samples do not require permeabilization.