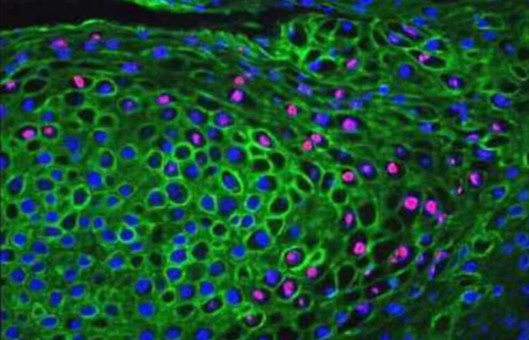
Protocols for IHC and Detection of Proliferating Cells by BrdU
GUIDELINE
Immunohistochemistry (IHC) is currently one of the most popular histological techniques, which is widely used to recognize and detect antigens in different tissues. The principle behind the IHC is the specific binding of antibodies to target antigenic epitopes and their subsequent detection.
The method exploits the principle that the target antigen is recognized by a specific antibody and is visualized using different detection systems. The subject of this protocol is the simultaneous immunohistochemical detection of protein antigens and proliferation marker BrdU in the developing tooth.
METHODS
- Inject 1 μl of BrdU solution per 1 g body weight into the timed pregnant mouse. Wait for 2 hours before euthanizing the animal.
- Dissect embryos in ice-cold PBS in a Petri dish under a dissection microscope, if necessary.
- Remove heads and transfer them into 24- or 48-well plates, wash in ice-cold PBS.
- Fix heads in 4% paraformaldehyde in PBS at 4°C for 1-2 h.
- Wash heads in PBS at 4°C overnight.
- Incubate heads in 30% sucrose in PBS at 4°C for 1-2 days.
- Dip heads into OCT for 1 min.
- Transfer heads to an embedding mold containing OCT. Orient heads as desired and freeze in ethanol, which contains dry ice. Store frozen specimen blocks at -80°C for several months.
- Attach the block to the specimen disc and cut 8- to 20- μm sections at -20°C in the cryostat. Thaw mount sections at room-temperature micro slides.
- Dry slides on a 40°C hot plate for 30-60 min. Slides can be placed in a slide box and stored at -80°C for several days.
- Wash sections 3 × with PBS, 10 min each time (to remove OCT). Rinse sections with cold methanol at -20°C for 2-3 min.
- Add 200 μl per slide of blocking buffer. Cover the slides with Para film coverslips carefully and incubate for 1 h.
- Carefully remove Para film coverslips and excess buffer from slides. Add 120 μl per slide of primary antibodies against antigenic protein diluted in blocking buffer. Cover the slides with Para film coverslips carefully. Incubate the slides at 4°C overnight.
- Wash sections 3 × with PBST, 10 min each time. Add 120 μl per slide of blocking buffer with species-specific cyanine-labeled secondary antibodies, which bind primary antibodies against the antigenic protein. Cover the slides with Para film coverslips carefully. Incubate the slides for 2 h.
- Wash sections 3 × with PBST, 10 min each time. Fix sections with 4% paraformaldehyde in PBS for 5 min.
- Wash sections 3 × with PBS, 5 min each time. Transfer the slide rack to the air incubator at 37°C. Add 2 N HCl and incubate the slides for 30 min.
- Wash sections with 0.1 M borate buffer, pH 8.5, for 15 min.
- Wash sections 3 × with PBS, 5 min each time. Add 200 μl per slide of blocking buffer. Cover the slides with Para film coverslips carefully and incubate for 1 h.
- Carefully remove Para film coverslips and excess buffer from the slides. Add 120 μl per slide of anti-BrdU antibodies diluted in blocking buffer. Cover the slides with Parafilm coverslips carefully. Incubate the slides at 4°C overnight.
- Wash sections 3 × with PBST, 10 min each time. Add 120 μl per slide of blocking buffer with species fi cyanine-labeled secondary antibodies, which bind anti-BrdU. Cover the slides with Para film coverslips carefully. Incubate the slides for 2 h.
- Wash sections 3 × with PBST, 10 min each time. Transfer the slides to a slide rack. Wash the sections 2 × with dH2O, 3 min each time.
- Dehydrate sections 1 × in 50, 70, and 95% ethanol in water, and 2 × in 100% ethanol, 3 min each time. Incubate sections 2 × in xylene for 3 min.
- Mount slides with DPX and apply micro cover glasses, being careful not to trap any air bubbles. Let slides dry overnight.
Creative Bioarray Relevant Recommendations
- Creative Bioarray offers a comprehensive IHC service from project design, and marker selection to image completion and data analysis. We are dedicated to satisfying every customer and assisting them to achieve their specific research goals.
- We provide a variety of reagents for studying cell proliferation, which are used to monitor the dynamic growth of a cell population or to detect daughter cells in a growing population.
NOTES
- Make fresh 4% paraformaldehyde each time. Preparation should be carried out inside a fume hood. Store it at 4°C for up to 1 week.
- Incorporation of BrdU can be detected in the thymus and bone marrow in as little as 1 h post-injection and 2 h in the tooth. At 24 h post-injection, BrdU can be detected in most tissues.
- Make sure that most of the blood is washed away (blood inhibits fixation). Heads of older embryos can be cut in half sagittal at midline using the razor blade. Each half can be embedded separately.
- The fixation timing should be optimized for each tissue individually, for example, 2 h for E18.5 heads, 1 h 30 min for E16.5 heads, 1 h 15 min for E14.5 heads, etc.