Multicolor FISH Protocol
GUIDELINE
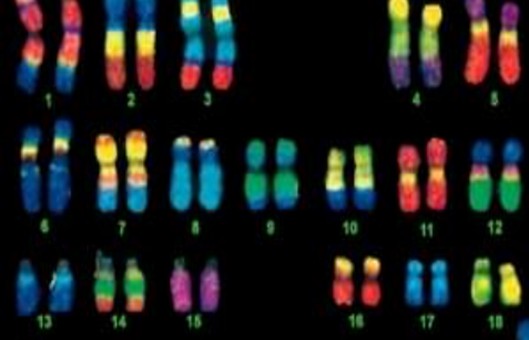
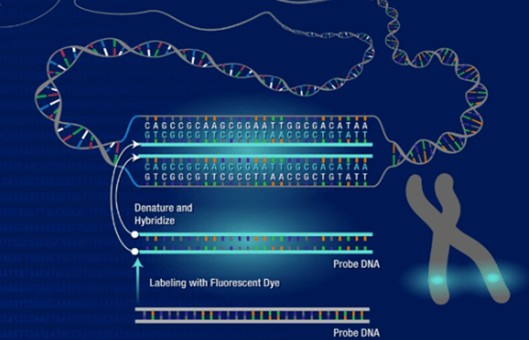
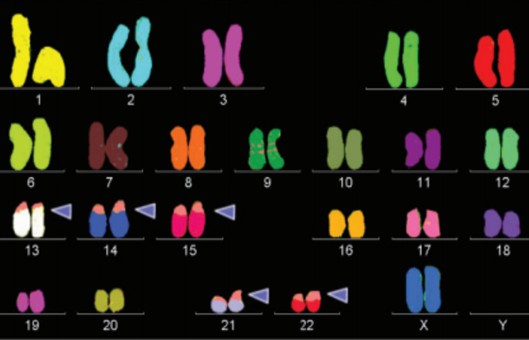
- Multicolor FISH (mFISH) is a method to facilitate the analysis of every single chromosome or chromosome part of a metaphase. Thus, marker chromosomes, complex chromosomal rearrangements, and all numerical aberrations can be visualized simultaneously in a single hybridization experiment.
- mFISH assays can be easily completed if the reagents are purchased commercially. If the investigator plans to produce the probes and antibody mixtures in-house, many more experiments and quality-control experiments will be required.
METHODS
- Prepare chromosome spreads.
- Under phase-contrast microscopy, determine the extent of cytoplasmic residue on the slide preparation.
- Dehydrate the slide in a 70%, 80%, and 100% ethanol series, 5 min each, and allow it to air dry.
- Apply 20 µl of the RNase A working solution to the slide and coverslip. Incubate for 30 min at 37°C.
- Remove the coverslip and wash the slide in 2×SSC for 5 min.
- Add 10 to 15 µl of 10% pepsin to 50 ml prewarmed 0.01 M HCl and treat the slide for 5 min. Then wash the slide in 1×PBS for 5 min.
- Incubate the slide in 1% formaldehyde/1×PBS/50 mM MgCl2 for 10 min at room temperature in a well-ventilated area or fume hood.
- Wash the slide in 1×PBS for 5 min at room temperature.
- Pass the slide through a 70%, 80%, and 100% ethanol series, 5 min each, and allow the slide to air dry.
- Denature the slide for 1.5 to 2 min in 70% formamide/2×SSC at 72°C.
- Promptly place the slide into 70% ethanol following denaturation and proceed through the dehydration series.
- Remove the slide from the ethanol, add the recommended amount of denatured and the pre-annealed commercial probe or 15 to 20 µl of the in-house probe, and hybridize for 48 hr at 37°C.
- After 48 hr, carefully peel off rubber cement from the slide and immerse in the first Coplin jar of 50% formamide/2×SSC at 45°C. Allow the coverslip to fall off and let stand for 5 min. Remove the slide and transfer to the second Coplin jar for 5 min. Repeat with the third solution.
- Wash the slide three times in three separate Coplin jars of 1×SSC, 5 min each wash, at 45°C.
- Remove the coverslip and wash slide in three washes of 0.1% Tween-20/4×SSC, 5 min each wash, at 45°C with gentle agitation.
- Drain excess solution, but do not allow the slide to dry, and add 40 µl of DAPI antifade counterstain. Apply coverslip and seal with clear nail polish. However, do not seal with nail polish if there are plans to re-probe the slide.
- Analyze the slide using fluorescence microscopy with the appropriate filters and imaging system capable of integrating multiple fluorochromes.
Creative Bioarray Relevant Recommendations
- M-FISH is defined as the simultaneous use of at least three different ligands or fluorescent dyes to specifically label DNA-excluding counterstains. Our M-FISH service provides the following 2 optional services, including whole chromosome, and specific chromosome M-FISH services.
- We also are dedicated to offering a portfolio of chromosome probes for our customers to detect the amplification, deletion, and translocation of genes and improve chromosome studies, which contain whole chromosome painting probes, centromere probes, sub-telomere-specific probes, satellite enumeration probes.
NOTES
- The incubations should use solutions in Coplin jars.
- Store the slides at -20°C when not in use.
RELATED PRODUCTS & SERVICES
For research use only. Not for any other purpose.
Resources
- FAQ
- Protocol
- Cell Culture Guide
- Technical Bulletins
-
Explore & Learn
-
Cell Biology
- How to Handle Mycoplasma in Cell Culture?
- How to Isolate PBMCs from Whole Blood?
- CHO Cell Line Development
- Troubleshooting Cell Culture Contamination: A Comprehensive Guide
- Contamination of Cell Cultures & Treatment
- Generation and Applications of Neural Stem Cells
- Stem Cell Markers
- Comparison of the MSCs from Different Sources
- Quantification of Cytokines
- Organoid Differentiation from Induced Pluripotent Stem Cells
- T Cell Activation and Expansion
- How to Isolate and Analyze Tumor-Infiltrating Leukocytes?
- Mesenchymal Stem Cells: A Comprehensive Exploration
- What are the Differences Between M1 and M2 Macrophages?
- What Cell Lines Are Commonly Used in Biopharmaceutical Production?
- Tips For Cell Cryopreservation
- Cryopreservation of Cells Step by Step
- What are PBMCs?
- STR Profiling—The ID Card of Cell Line
- Comparison of Several Techniques for the Detection of Apoptotic Cells
- Enrichment, Isolation and Characterization of Circulating Tumor Cells (CTCs)
- Strategies for Enrichment of Circulating Tumor Cells (CTCs)
- How to Assess the Migratory and Invasive Capacity of Cells?
- How to Decide Between 2D and 3D Cell Cultures?
- Isolation, Expansion, and Analysis of Natural Killer Cells
- Neural Differentiation from Induced Pluripotent Stem Cells
- How to Eliminate Mycoplasma From Cell Cultures?
- Monocytes vs. Macrophages
- How to Detect and Remove Endotoxins in Biologics?
- Comparison of Different Methods to Measure Cell Viability
- What Are Myeloid Cell Markers?
- How to Start Your Culture: Thawing Frozen Cells
- Biomarkers and Signaling Pathways in Tumor Stem Cells
- Techniques for Cell Separation
- Circulating Tumor Cells as Cancer Biomarkers in the Clinic
- CFU Assay for Hematopoietic Cell
- Guidelines for Cell Banking to Ensure the Safety of Biologics
- Cell Cryopreservation Techniques and Practices
- Cell Culture Medium
- T Cell, NK Cell Differentiation from Induced Pluripotent Stem Cells
- Major Problems Caused by the Use of Uncharacterized Cell Lines
- Critical Quality Attributes and Assays for Induced Pluripotent Stem Cells
- Multi-Differentiation of Peripheral Blood Mononuclear Cells
- Human Primary Cells: Definition, Assay, Applications
- What are Mesothelial Cells?
- How to Scale Up Single-Cell Clones?
- Unveiling the Molecular Secrets of Adipogenesis in MSCs
- Tumor Stem Cells: Identification, Isolation and Therapeutic Interventions
- Direct vs. Indirect Cell-Based ELISA
- What Is Cell Proliferation and How to Analyze It?
- IL-12 Family Cytokines and Their Immune Functions
- Spheroid vs. Organoid: Choosing the Right 3D Model for Your Research
- From Collection to Cure: How ACT Works in Cancer Immunotherapy
- Mastering Cell Culture and Cryopreservation: Key Strategies for Optimal Cell Viability and Stability
- Adherent and Suspension Cell Culture
- Understanding Immunogenicity Assays: A Comprehensive Guide
- How to Maximize Efficiency in Cell-Based High-Throughput Screening?
- What are White Blood Cells?
- Immunogenicity Testing: ELISA and MSD Assays
- Role of Cell-Based Assays in Drug Discovery and Development
- Types of Cell Therapy for Cancer
- 3D-Cell Model in Cell-Based Assay
- Immunogenicity Testing: ELISA and MSD Assays
- What Are the Pros and Cons of Adoptive Cell Therapy?
- Eosinophils vs. Basophils vs. Neutrophils
- 3D-Cell Model in Cell-Based Assay
- Cultivated Meat: What to Know?
- Exploring Cell Dynamics: Migration, Invasion, Adhesion, Angiogenesis, and EMT Assays
- From Blur to Clarity: Solving Resolution Limits in Live Cell Imaging
- A Complete Guide to Immortalized Cancer Cell Lines in Cancer Research
- Cell Viability, Proliferation and Cytotoxicity Assays
- What Are CAR T Cells?
- Live Cell Imaging: Unveiling the Dynamic World of Cellular Processes
- Optimization Strategies of Cell-Based Assays
- Overview of Cell Apoptosis Assays
- Cell-Based High-Throughput Screening Techniques
- Key Techniques in Primary, Immortalized and Stable Cell Line Development
- From Primary to Immortalized: Navigating Key Cell Lines in Biomedical Research
- From Blur to Clarity: Solving Resolution Limits in Live Cell Imaging
- Optimization Strategies of Cell-Based Assays
- Cell Immortalization Step by Step
- Live Cell Imaging: Unveiling the Dynamic World of Cellular Processes
-
Histology
- Fluorescent Nuclear Staining Dyes
- Troubleshooting in Fluorescent Staining
- Immunohistochemistry Controls
- Overview of the FFPE Cell Pellet Product Lines
- Guides for Live Cell Imaging Dyes
- Tips for Choosing the Right Protease Inhibitor
- Instructions for Tumour Tissue Collection, Storage and Dissociation
- Mitochondrial Staining
- How to Apply NGS Technologies to FFPE Tissues?
- Stains Used in Histology
- Multiple Animal Tissue Arrays
- Cell and Tissue Fixation
- Immunohistochemistry Troubleshooting
- Overview of Common Tracking Labels for MSCs
- Cell Lysates: Composition, Properties, and Preparation
- Comparison of Membrane Stains vs. Cell Surface Stains
- Microscope Platforms
- How to Choose the Right Antibody for Immunohistochemistry (IHC)
- How to Begin with Multiplex Immunohistochemistry (mIHC)
- Common Immunohistochemistry Stains and Their Role in Cancer Diagnosis
- Serum vs. Plasma
- Comparing IHC, ICC, and IF: Which One Fits Your Research?
- Modern Histological Techniques
- Multiplexing Immunohistochemistry
- What You Must Know About Neuroscience IHC?
- From Specimen to Slide: Core Methods in Histological Practice
- How Immunohistochemistry Makes the Invisible Brain Visible?
- Histological Staining Techniques: From Traditional Chemical Staining to Immunohistochemistry
-
Exosome
- How to Enhancement Exosome Production?
- Classification, Isolation Techniques and Characterization of Exosomes
- Emerging Technologies and Methodologies for Exosome Research
- How to Label Exosomes?
- How to characterize exosomes?
- How to Perform Targeted Modification of Exosomes?
- Techniques for Exosome Quantification
- Exosomes as Emerging Biomarker Tools for Diseases
- How to Apply Exosomes in Clinical?
- How to Efficiently Utilize MSC Exosomes for Disease Treatment?
- Exosome Transfection for Altering Biomolecular Delivery
- Summary of Approaches for Loading Cargo into Exosomes
- What's the Potential of PELN in Disease Treatment?
- How do PELN Deliver Drugs?
- Current Research Status of Milk Exosomes
- Collection of Exosome Samples and Precautions
- How Important are Lipids in Exosome Composition and Biogenesis?
- Common Techniques for Exosome Nucleic Acid Extraction
- What are the Functions of Exosomal Proteins?
- The Role of Exosomes in Cancer
- Exosome Size Measurement
- Exosome Quality Control: How to Do It?
- Applications of MSC-EVs in Immune Regulation and Regeneration
- Unraveling Biogenesis and Composition of Exosomes
- Exosome Antibodies
- Production of Exosomes: Human Cell Lines and Cultivation Modes
-
ISH/FISH
- ISH probe labeling method
- CARD-FISH: Illuminating Microbial Diversity
- Comprehensive Comparison of IHC, CISH, and FISH Techniques
- RNAscope ISH Technology
- Comparative Genomic Hybridization and Its Applications
- What are the Differences between FISH, aCGH, and NGS?
- FISH Tips and Troubleshooting
- Small RNA Detection by ISH Methods
- What Is the Use of FISH in Solid Tumors?
- Mapping of Transgenes by FISH
- Overview of Oligo-FISH Technology
- Differences Between DNA and RNA Probes
- What are Single, Dual, and Multiplex ISH?
- FISH Techniques for Biofilm Detection
- Whole Chromosome Painting Probes for FISH
- Multiple Options for Proving Monoclonality
- Telomere Length Measurement Methods
- Overview of Common FISH Techniques
- Guidelines for the Design of FISH Probes
- Reagents Used in FISH Experiments
- Multiple Approaches to Karyotyping
- In Situ Hybridization Probes
- What Types of Multicolor FISH Probe Sets Are Available?
- How to Use FISH in Hematologic Neoplasms?
- Different Types of FISH Probes for Oncology Research
- ImmunoFISH: Integrates FISH and IL for Dual Detection
- 9 ISH Tips You Can't Ignore
-
Toxicokinetics & Pharmacokinetics
- Pharmacokinetics of Therapeutic Peptides
- Toxicokinetics vs. Pharmacokinetics
- Organoids in Drug Discovery: Revolutionizing Therapeutic Research
- What Are Metabolism-Mediated Drug-Drug Interactions?
- How to Improve Drug Plasma Stability?
- How Is the Cytotoxicity of Drugs Determined?
- How to Improve the Pharmacokinetic Properties of Peptides?
- How to Conduct a Bioavailability Assessment?
- Organ-on-a-Chip Systems for Drug Screening
- Pharmacokinetics Considerations for Antibody Drug Conjugates
- Key Considerations in Toxicokinetic
- Experimental Methods for Identifying Drug-Drug Interactions
- How to Improve Drug Distribution in the Brain
- Effects of Cytochrome P450 Metabolism on Drug Interactions
- Overview of In Vitro Permeability Assays
- Comparison of MDCK-MDR1 and Caco-2 Cell-Based Permeability Assays
- Traditional vs. Novel Drug Delivery Methods
- What factors influence drug distribution?
- How to Design and Synthesize Antibody Drug Conjugates?
- Methods of Parallel Artificial Membrane Permeability Assays
- Key Factors Influencing Brain Distribution of Drugs
- Unraveling the Role of hERG Channels in Drug Safety
- Predictive Modeling of Metabolic Drug Toxicity
- The Rise of In Vitro Testing in Drug Development
- Physical and Chemical Properties of Drugs and Calculations
- What Is the Role of the Blood-Brain Barrier in Drug Delivery?
- Parameters of Pharmacokinetics: Absorption, Distribution, Metabolism, Excretion
- What are the Pharmacokinetic Properties of the Antisense Oligonucleotides?
- What Are Compartment Models in Pharmacokinetics?
- The 8 Costliest Mistakes in Preclinical CYP Phenotyping
- When Should You Introduce ADME Tox Testing in Drug Development?
- 6 Easy Steps to Get Your In Vitro ADME Done
- From Cells to Systems: Modern Approaches to Disease Modeling
- How to Choose the Right In Vitro ADME Assays for Small-Molecule Drugs
- How Genotoxicity Testing Guides Safer Drug Development
- Top 5 Pitfalls in In Vitro ADME Assays and How to Avoid Them
- What Is Genotoxicity in Pharmacology? Mechanisms and Sources
- In Vitro ADME vs In Vivo ADME
- A Complete Guide to CYP Reaction Phenotyping in 2026
- How to Interpret CYP Phenotyping Data
- Reaction Phenotyping vs. Metabolic Stability
- What Are the Best Methods to Test Cardiotoxicity?
- Why Cardiotoxicity Matters in R&D?
-
Disease Models
- What Human Disease Models Are Available for Drug Development?
- Overview of Cardiovascular Disease Models in Drug Discovery
- Summary of Advantages and Limitations of Different Oncology Animal Models
- Animal Models of Neurodegenerative Diseases
- Preclinical Models of Acute Liver Failure
- Disease Models of Diabetes Mellitus
- Why Use PDX Models for Cancer Research?
-
Cell Biology
- Life Science Articles
- Download Center
- Trending Newsletter