GUIDELINE
- The Mouse Hematopoietic Stem Cell Long Cycle Culture System (LTC) can be used to detect and count primitive hematopoietic progenitor cells. The LTC culture system has also been used for the generation and quantification of lymphocytes.
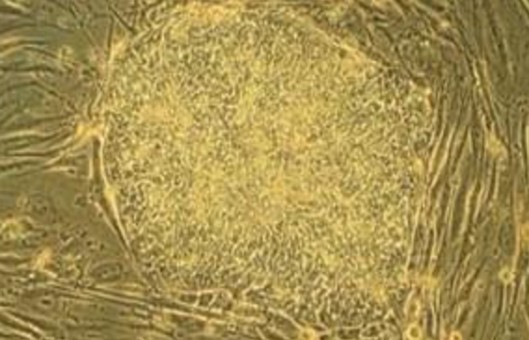
- The primary application of this culture system is the quantification of murine primitive hematopoietic progenitor cells that initiate and sustain myeloid hematopoiesis for several weeks in vitro. Because of the functional and phenotypic similarities between these hematopoietic progenitors and in vivo hematopoietic reconstituted stem cells, these cells are commonly referred to as long-term culture-initiating cells (LTC-IC).
- The unique characteristics of the long-term culture system led to the development of the LTC-IC assay, an assay capable of detecting and quantifying primitive hematopoietic progenitor cells with the same phenotypic and functional characteristics as in vivo hematopoietic reconstituted stem cells. In the mouse long-period culture system, colony-forming units (CFUs) detected after 24 weeks represent the progeny of LTC-IC, because, at this point in the inoculated cell suspension, LTC-IC have undergone terminal differentiation of CFUs.
- Detection of LTC-C quantification in cell suspensions requires co-culture of the assay cells with supporting feeder layer cells, which are either some irradiated stromal cells or a suitable mouse fibroblast cell line. The limiting release assay is used to stem determine the frequency of LTC-IC and the average number of CFUs produced per LTC-C. Once the average number of CFUs per LTC-IC is established, the LTC-IC content of the samples could be determined by bulk LTC-C culture experiments in which the source and culture conditions of the test cells passed in these LTC-IC culture experiments are identical. The LTC-IC content is then calculated by dividing the total number of CFUs by the average number of CFUs produced per LTC-IC.
METHODS
- Preparation of feeder layer cells.
- HLTM liquid dispensing.
- Inoculation of cells to be tested.
- Perform the assay 5-6 weeks after cell inoculation, and change the liquid half-volume every week.
- Collect cells for assay. The supernatant is discarded and 0.25% Trypsin is added for digestion. It is required to observe the digestion process under the microscope, and the digestion is terminated with trypsin digestion termination solution when the feeder layer has not been digested and the hematopoietic cells have been properly digested according to the principle that the digestion time of hematopoietic cells and feeder layer cells are different.
- Prepare three 35 mm Petri dishes to prepare a double culture system. Place two 35 mm Petri dishes with lids and one 35 mm uncovered petri dish containing water together into a 100 mm petri dish.
- Prepare CD34 cells, and after cell counter counting dilute the cells with 0.3 mL IMDM 2% FBS to 10 times the final concentration of the inoculum. The recommended cell count is 500 to 2000 cells per 3.5 cm culture dish. The diluted cell suspension is then added to the medium at a ratio of 1:10.
- The cells are shaken by vigorous vortex suspension immediately after transferring into the flow tube containing medium. Then let stand for 5 min to allow the air bubbles to dissipate.
- Attach a sterile 16-gauge blunt-ended needle to a sterile 3 mL syringe and aspirate the mixture of medium and cells with the syringe, adding 1.1 mL to each 3.5 cm Petri dish with the cap in place. Use a new set of blunt-ended needles and syringes for each inoculated tube of the mixture.
- Repeat the operation to inoculate the cells into another 35 mm culture dish.
- Gently tilt and rotate the culture dish so that the culture solution is spread out and evenly distributed within the dish.
- Place the 35 mm culture dish with inoculated cells in the 10 cm culture dish and add 3 mL of sterile water to the 35 mm culture dish without the lid and put the lid on the 10 cm culture dish.
- The Petri dishes are placed in a CO2 incubator and incubated for 14 to 16 days.
- After 14 to 16 days of incubation, clone counting is performed.
Creative Bioarray Relevant Recommendations
NOTES
- The optimal inoculation concentration may vary depending on the mouse strain and the hematopoietic stem and progenitor cell enrichment procedure used and should be optimized in each laboratory to establish the optimal inoculation concentration.
- Wells will be scored as positive if one or more colonies are detected, or negative if no colonies are observed.
RELATED PRODUCTS & SERVICES