FISH Protocol for Metaphase Chromosomes
GUIDELINE
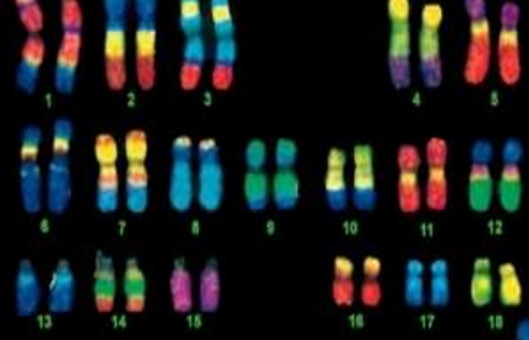
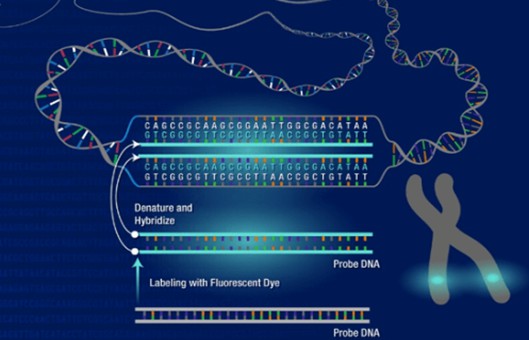
Fluorescence in situ hybridization (FISH) has become a powerful, practical tool for detecting genetic abnormalities such as chromosome translocations, pericentric inversions, and insertions. Most frequently, chromosomes are studied as mitotic metaphases fixed on slides. Here, we describe a basic methodology for the accomplishment of metaphase fluorescence in situ hybridization (FISH).
METHODS
Pretreatment of metaphase chromosomes for FISH
- Equilibrate the slide by briefly dipping in 2xSSC.
- Digest in pre-warmed pepsin solution and incubate at 37°C for 10 minutes in a shaking water bath.
- Wash 2x5 mins in 1xPBS at room temperature on a shaker.
- Wash for 3 minutes in 1x PBS-Magnesium Chloride at room temperature on a shaker.
- Pre-fixing. Incubate the slide in 70 ml PBS-Magnesium chloride containing 2 ml 37% Formaldehyde for 5 minutes at room temperature (no shaking).
- Wash 5 minutes in 1x PBS on a shaker.
- Dehydrate the slide by dipping it in Ethanol series (3 minutes each in 70%, 90%, and 100% Ethanol).
- Allow the slide to air dry.
- Place the Ethanol series at -20°C.
- Pre-warm the slides to 60°C.
- Once the slide is dry, and working quickly, add 100 μl of pre-warmed (75°C) denaturation mix on the area of the slide marked with good metaphase chromosomes, and place a 24 x 50 mm coverslip over the slide and incubate it in a steel container with lid for 1 minute 45 seconds at 72-75°C or on a heated PCR block (75°C).
- Remove the cover slip immediately and dip the slide briefly in ice-cold 2xSSC.
- Dip the slides in ice-cold Ethanol series (3 minutes each in ice-cold 70%, 90%, and 100% Ethanol).
- Allow the slide to air dry.
- Slides are ready for hybridization.
FISH
- To air-dried precipitated DNA probe, add 6 μl de-ionized formamide and incubate it for about 1 hour at 37°C in a thermos-shaker (under shaking conditions).
- Add 6 μl of hybridization buffer and mix thoroughly and carefully.
- Continue incubation without shaking for an additional 15 minutes.
- Denature the DNA by incubating for 5 minutes at 75°C.
- Place immediately on ice for about 5 minutes. (If the probe is meant for repetitive sequences, pre-anneal the DNA by incubating the probe at 37°C for 40 minutes).
- Place the probe carefully on the slide area marked with good metaphase chromosomes and place an 18x18mm coverslip over the probe, seal around the coverslip with rubber cement, and place it in a humidified chamber.
- Hybridize overnight at 37°C in a humidified incubator.
Creative Bioarray Relevant Recommendations
- Compared with interphase, metaphase chromosomes have certain characteristics of intergenerational and genetic structure. Creative Bioarray provides Metaphase FISH Services, including experimental design, optional metaphase chromosome preparation, FISH probe customization, FISH hybridization, microscope services, and data services. Our platform can complete all steps from sample pre-processing, and work with you to find a FISH solution that meets your needs.
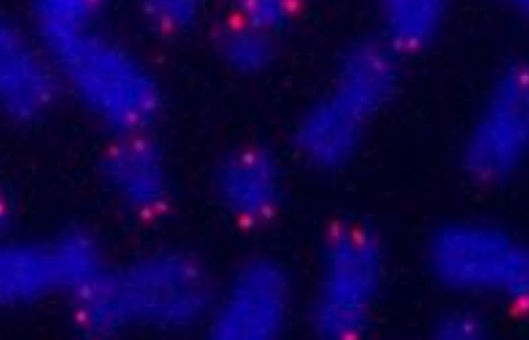
Detection
- Remove the rubber cement and cover slip carefully and discard.
- Incubate slide for 10mins in 2xSSC at 37°C in a shaking water bath.
- Incubate slide 2x7 mins in 0.2xSSC at 53°C in a shaking water bath.
- Briefly equilibrate slides in 4xSSC/Tween 20.
- Add 150 μl of blocking solution on the coverslip, and place the slide carefully over the coverslip. Place the slide and coverslip in a rack with the coverslip facing down, place the rack in a humidified metal container, and incubate at 30°C for 30 minutes.
- Carefully remove and discard the coverslip. Wash briefly in 4xSSC/Tween 20 (45°C).
- Incubate with 150 μl of antibody I as before at 37°C for 30 minutes.
- Carefully remove and discard the coverslip. Wash 3x5 mins in 4xSSC/Tween 20 (45°C).
- Incubate with 150 μl of antibody II as before at 37°C for 45 minutes.
- Carefully remove and discard the coverslip. Wash 3x5 mins in 4xSSC/Tween 20 (45°C).
- Incubate with 150 μl of antibody III as before at 37°C for 30 minutes.
- Carefully remove and discard the coverslip. Wash 3x5 mins in 4xSSC/Tween 20 (45°C).
- Incubate for 2-10 minutes in DAPI solution in the dark.
- Wash 5xin distilled water. Air dry in the dark.
- Add two drops of anti-fade on the slide and place a 24x50 mm coverslip over it.
- Store in the dark at 4°C.
NOTES
- One probe may contain more troublesome repetitive sequences, have a higher annealing temperature, or prove more resistant to restriction enzyme digestion. If one probe provides unsatisfactory results, another should be tried until one is found that can provide consistent, reliable hybridization results.
- When preparing metaphase chromosomes for FISH (or banding), all pipetting manipulations must be accomplished very gently to avoid disruption of nuclear membranes and dispersal of chromosomes before slide preparation. Otherwise, an insufficient number of metaphase spreads and/or incomplete metaphase spreads will result.