Construction and Culture Protocol of Neural Stem Cells
GUIDELINE
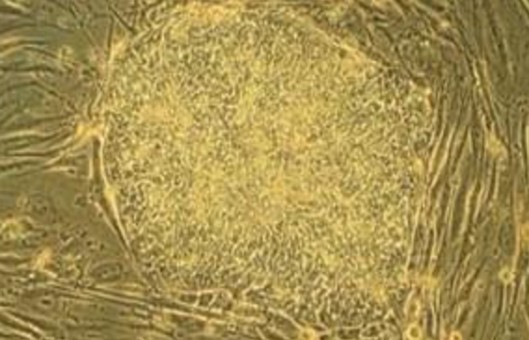
Because neural stem cells (NSCs) have the potential of self-renewal and multidirectional differentiation, the method of suspended neural bulb culture can be used to obtain and study. After processing the embryonic brain tissue into a single cell, only the cells with the ability of self-renewal can be cloned and proliferated into a suspended nerve ball in the culture medium, and with the progress of subculture, the ability of proliferation and differentiation into a variety of neural progeny cells can be maintained.
METHODS
- Transfer the culture medium containing nerve balls into a 15 mL conical tube.
- Clean the plate with a few of DPBS to maximize cell recovery.
- Centrifuge at 5-7 g for 100-130 minutes according to the size of the global nerve bulb.
- Carefully suck out the supernatant and add 200 μL of solution. Gently re-suspend the cell precipitates by pipetting 10-200 times with a p5 micropipette.
- Incubate at room temperature for 10 minutes.
- Dilute with 800 μL of complete medium and use p10 micropipette suction to thoroughly move the solution up and down 15-1000 times to avoid bubble formation.
- The cell suspension is adjusted to 8-10 mL of control culture medium. The plates are gently inverted 1-2 times, centrifuged at 10 g for 200 minutes, and mixed carefully. The supernatant is aspirated, and the resulting precipitate is re-suspended in 1000 mL of complete culture medium. This optional washing will reduce the presence of dead cells in the suspension and is especially recommended for the first 1-4 generations.
- Depending on the original number and size of the nerve balls, it may be necessary to dilute the resulting cell suspension with a complete culture medium to achieve a density that ensures a reliable cell count.
- Take a small sample and estimate the concentration of live cells, or use an automated cell counter system if available.
- 10,000 live cells /cm2 are used for culture, and subculture and batch expansions are performed on fresh preheated complete culture medium.
- Incubate in a 5% CO2 humidifier incubator at 37°C. The new nerve balls should be ready for passage after 5-7 days. Healthy cultures should be expanded in a ratio of 1:3 to 1:5.
NOTES
- Once primary nerve balls have been obtained from SEZ tissues, their amplification time can be extended by dissociating them after 4-7 days and inoculating individual cells under the same conditions. The culture can be passaged more than 25 times without loss of proliferation potential.
- The protocol originally published relies on mechanical decomposition of the nerve bulb by repeatedly passing the suspension through the p200 micropipette suction head. In experience, while this method can be used, it can result in partially dissociated nerve balls if the experimenter is not well trained. In addition, complete mechanical depolymerization requires up to a hundred petting times, which may stress the cells and lead to a loss of vitality.
- The proliferation ability of cells is significantly related to the age of the tissue source animal and the brain region taken. The younger the gestational age, the more immature the developmental state of stem cells, the stronger the proliferation ability of cells. Cortical derived neural stem cells are more likely to obtain cells with good proliferation ability than other brain regions.
- Meningeal tissue can induce adhesion and differentiation of neural stem cells, so meningeal tissue must be cleared during tissue treatment.
- Serum can induce differentiation of kilo nuclear stem cells, so the culture medium with serum should not be used when the digestion is terminated.
- The passage time is generally about 7-10 days, but if the volume of the nerve ball is large, or the center of the nerve ball has begun to appear black areas under the light microscope (the reason for the black area may be the volume of the ball is too large, affecting the light transmission, but at this time the center of the ball often appears dead cells), so the passage should be timely.