Tau Aggregation Assay
Tau is separated from the microtubules, causing itself to be unstable. It usually aggregates into oligomers, pairs of helical filaments (PHF), and eventually neurofibrillary tangles. Abnormal aggregation of tau into PHF is one of main hallmarks of Alzheimer's disease. Aggregation occurs in the cytoplasm and may be cytotoxic to neurons. Therefore, compounds that inhibit tau aggregation could provide a basis for the development of tools for the therapy of tau pathology in AD.
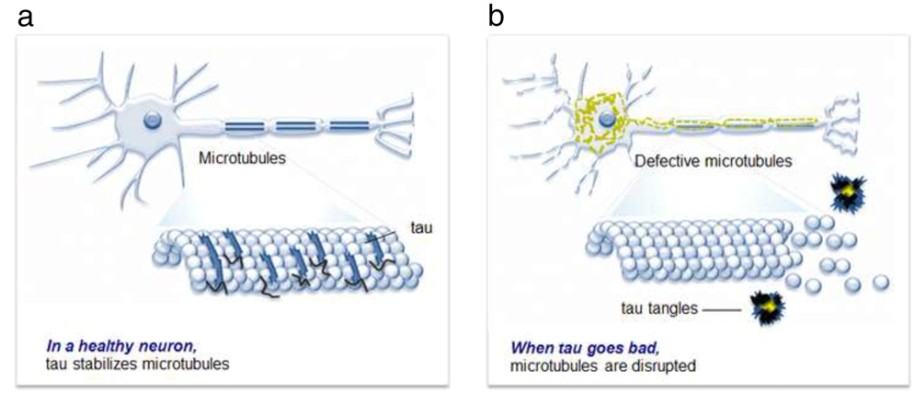 Figure 1 Tau aggregation and neuronal degeneration. (a) In a healthy neuron, tau stabilizes microtubules promoting axonal outgrowth and synaptic vesicle transport. (b) When tau goes bad, tau becomes neurotoxic aggregates and microtubules become dissociates.
Figure 1 Tau aggregation and neuronal degeneration. (a) In a healthy neuron, tau stabilizes microtubules promoting axonal outgrowth and synaptic vesicle transport. (b) When tau goes bad, tau becomes neurotoxic aggregates and microtubules become dissociates.
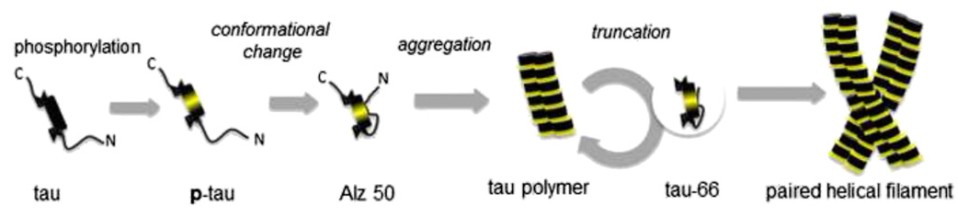 Figure 2 Diagrammatic representation of tau aggregation
Figure 2 Diagrammatic representation of tau aggregation
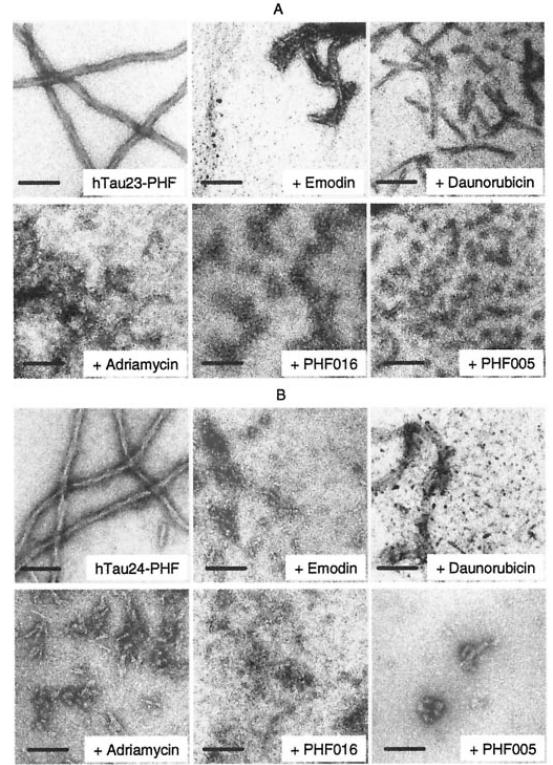 Figure 3 Electron microscopy of PHFs. PHFs were assembled from htau23 (A, three repeats, no inserts) or htau24 (B, four repeats, no inserts) at 10 μM protein concentration, 2.5 μM heparin in PBS buffer, pH 7.4, 37 °C. After assembly the inhibitor compounds were added at 60 μM, and the time course of disassembly was monitored by electron microscopy. The examples show breakdown products after overnight incubation with inhibitors. Bar, 100 nm.
Figure 3 Electron microscopy of PHFs. PHFs were assembled from htau23 (A, three repeats, no inserts) or htau24 (B, four repeats, no inserts) at 10 μM protein concentration, 2.5 μM heparin in PBS buffer, pH 7.4, 37 °C. After assembly the inhibitor compounds were added at 60 μM, and the time course of disassembly was monitored by electron microscopy. The examples show breakdown products after overnight incubation with inhibitors. Bar, 100 nm.
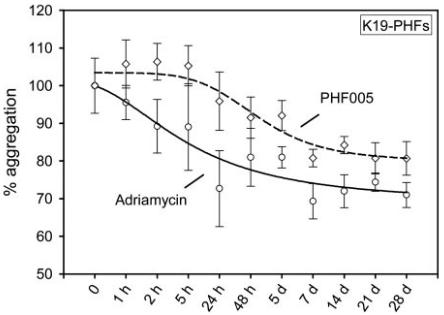 Figure 4 Time course of PHF disassembly (ThS assay) at low inhibitor concentrations. PHFs were formed as above (10 μM construct K19, 2.5 μM heparin, overnight) and then exposed to 0.5 μM adriamycin (solid line) or PHF005 (dashed line). The degree of assembly was measured by the ThS assay. Note that despite the low inhibitor concentrations there is a gradual decrease of PHFs. Untreated controls were measured in parallel and subtracted as background.
Figure 4 Time course of PHF disassembly (ThS assay) at low inhibitor concentrations. PHFs were formed as above (10 μM construct K19, 2.5 μM heparin, overnight) and then exposed to 0.5 μM adriamycin (solid line) or PHF005 (dashed line). The degree of assembly was measured by the ThS assay. Note that despite the low inhibitor concentrations there is a gradual decrease of PHFs. Untreated controls were measured in parallel and subtracted as background.
Creative Bioarray offers diverse customized in vitro models to help our customers screen compounds that can inhibiting tau aggregation. These in vitro models, including primary neuronal cultures and cell lines, are phenotypically closer to those observed in the aged neuronal network and mimic the development of AD.
We provide this powerful and versatile tool for developing potential AD therapy directly as well as basic research to help our customers who wish to understand more about the underlying mechanisms of AD from which they could facilitate the process of drug development.
Quotation and ordering
Contact us if you have any questions. Our customer service representatives are available 24hr a day.
References
- Lim S.; et al. Cell-based models to investigate tau aggregation. Comput Struct Biotec. 2014, 12(20-21): 7-13.
- Pickhardt M.; et al. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. J Biol Chem, 2005, 280(5): 3628-3635.
For research use only. Not for any other purpose.
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Oral Mucositis Model
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Graft-versus-host Disease (GvHD) Models
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Schizophrenia Model
- Depression Models
- Pain Models
-
Metabolic Disease Models
- Type 1 Diabetes Mellitus Model
- Type 2 Diabetes Mellitus Model
- Animal Model of Hyperuricemia
-
Nonalcoholic Fatty Liver Disease Model
- High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Methionine and Choline Deficient (MCD) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Gubra-Amylin NASH (GAN) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Streptozotocin (STZ) Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- High Fat Diet-Induced Obesity Model
- Diabetic Foot Ulcer (DFU) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthotopic Kidney Transplantation Model
- Benign Prostatic Hyperplasia (BPH) Model
- Peritoneal Fibrosis Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
- Otology Disease Models