Aβ Induced Model
Senile plaques are one of the most important histopathological hallmarks of Alzheimer’s disease which were composed by amyloid β-peptides, 36-43 amino acid peptide fragments of β-amyloid precursor protein. The amyloid hypothesis of Alzheimer’s disease suggests that amyloid β-peptides triggers a neurotoxic cascade, which usually results in neurodegeneration and AD.
Creative Bioarray specializes in providing customized pharmacodynamic research services to help customers assess the efficacy of drug candidates and study the associated pathological mechanisms through Aβ induced model.
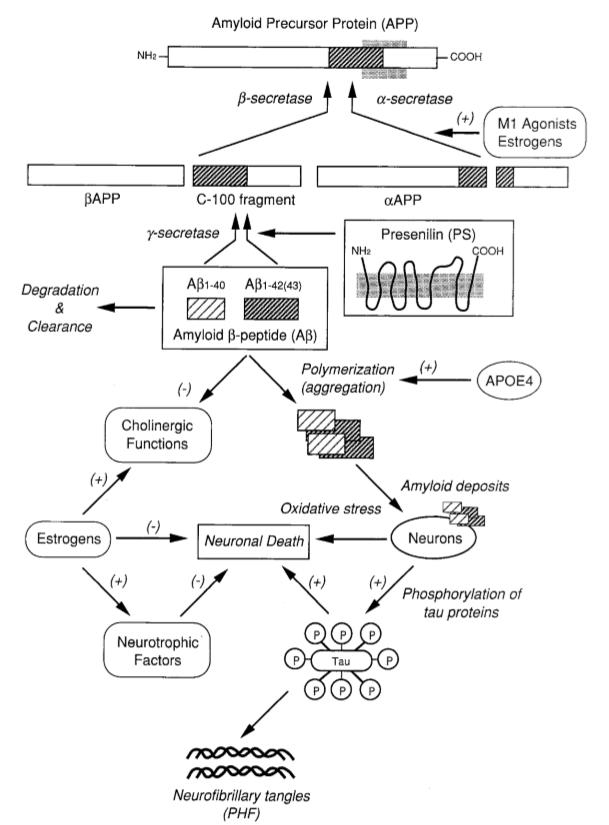 Figure. 1. The amyloid cascade hypothesis of Alzheimer's disease and its pharmacological modulation.
Figure. 1. The amyloid cascade hypothesis of Alzheimer's disease and its pharmacological modulation.
Our capabilities
- We can provide comprehensive animal models to meet your needs for pre-clinical drug candidate screening and mechanism research.
- We can provide comprehensive behavioral and cognitive testing on AD models for drug screening.
- We can evaluate various biomarkers through WB, IHC, ELISA, sequencing, etc.
- We can utilize LTP (long-term potentiation) assay to evaluate your preclinical drug candidates against the synaptic impairments in animals.
- We can evaluate anti-oxidative stress in the brains of animals treated with drug candidates.
Assays available
- Learning and memory deficits tests
- Synaptic impairment
- Oxidative stress
- Neuroinflammation
- Phosphorylated tau
- Glycogen synthase kinase-3 beta (GSK3β)
- Neurofilament Light Chain levels
- Neuronal loss
- β-AP level
- Plaque load
- β-sheet load
- pE(3)-Aβ load
- Enzyme activity related to cholinergic system
- NMDA receptor function and excitotoxicity
- Mitochondrial dysfunction
- Brain slice staining and synaptic electrophysiology
- Blood brain barrier homeostasis
- Cerebral vascular angiopathy (CAA)
Our team of experts has many years of experience with AD models and is ready to meet challenges and help you make informed decisions.
Study examples
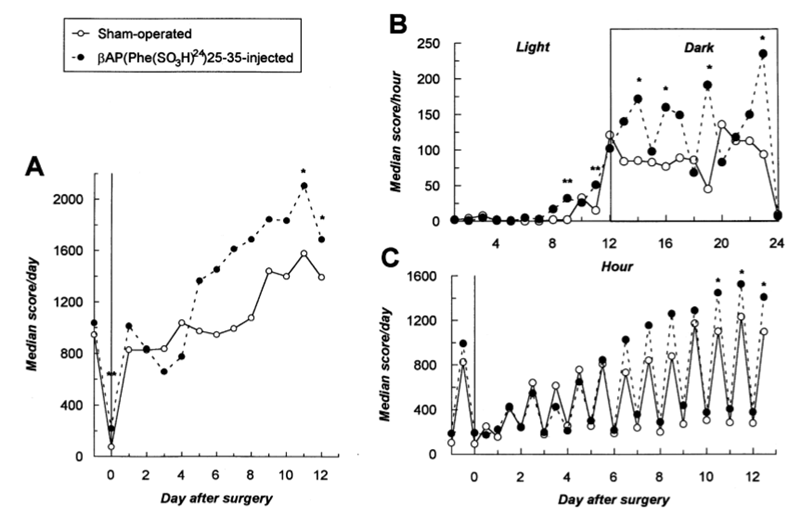 Fig. 2. Changes in home cage activity of βAP(Phe(SO3H)24)25-35-lesioned and sham-operated animals up to 13 days post-injection (a). Note the biphasic activity profile of βAP(Phe(SO3H)24)25-35-treated rats. Although hypoactivity occurred transiently in the initial phase of the experiment (day 2-4 post-surgery); βAP-injected animals developed a persistent hyperactivity, reaching the signficant level on day 12 post-injection. Detailed analysis of motor activities assessed 12 days after βAP(Phe(SO3H)24)25-35 infusions (b) revealed a significant increase in ambulation of βAP-injected rats. βAP(Phe(SO3H)24)25-35 treatment did not alter behavioral reactivities in an illuminated environment and thus the peptide did not affect the circadian rhythm of the animals (c). Illuminated conditions were maintained from 08.00 to 20.00 h. ** P<0.01, * P<0.05 (Kruskal–Wallis test), relative to the sham-operated group. Data are presented as medians; n=10 [βAP lesioned] and n=9 [sham].
Fig. 2. Changes in home cage activity of βAP(Phe(SO3H)24)25-35-lesioned and sham-operated animals up to 13 days post-injection (a). Note the biphasic activity profile of βAP(Phe(SO3H)24)25-35-treated rats. Although hypoactivity occurred transiently in the initial phase of the experiment (day 2-4 post-surgery); βAP-injected animals developed a persistent hyperactivity, reaching the signficant level on day 12 post-injection. Detailed analysis of motor activities assessed 12 days after βAP(Phe(SO3H)24)25-35 infusions (b) revealed a significant increase in ambulation of βAP-injected rats. βAP(Phe(SO3H)24)25-35 treatment did not alter behavioral reactivities in an illuminated environment and thus the peptide did not affect the circadian rhythm of the animals (c). Illuminated conditions were maintained from 08.00 to 20.00 h. ** P<0.01, * P<0.05 (Kruskal–Wallis test), relative to the sham-operated group. Data are presented as medians; n=10 [βAP lesioned] and n=9 [sham].
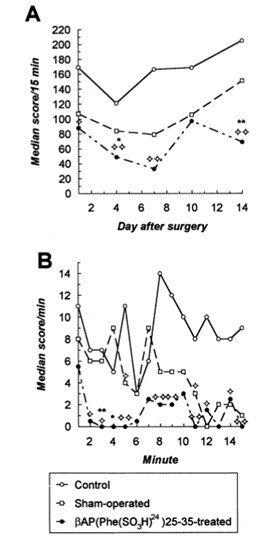 Fig. 3. Effects of βAP(Phe(SO3H)24)25-35 injected in the rat nmb on the locomotor activity 1, 4, 7, 10 and 14 days post-surgery (a). In the experiments ten βAP(Phe(SO3H)24)25-35-lesioned, nine shamoperated and eight naive control animals were used. βAP(Phe(SO3H)24)25-35-treated rats showed a significant hypoactivity in the locomotor arena throughout the four test sessions, compared to both sham-operated and naive control groups. However, the explorative behavior of sham-operated animals was, albeit non-significantly, also decreased. Detailed analysis of locomation 7 days post-injection (c) revealed a dramatic reduction in horizontal ambulation and rearing activities of βAP(Phe(SO3H)24)25-35 injected rats during the initial phase of the tests. There are obvious differences between the activity profiles of βAP(Phe(SO3H)24)25-35-treated and sham-operated or naive control animals. * P<0.05, ** P<0.01 vs. the sham-operated group, while significant differences between βAP(Phe(SO3H)24)25-35 lesioned and control animals are indicated as follows: P<0.01, P<0.05 (Kruskal–Wallis test). Data are presented as medians.
Fig. 3. Effects of βAP(Phe(SO3H)24)25-35 injected in the rat nmb on the locomotor activity 1, 4, 7, 10 and 14 days post-surgery (a). In the experiments ten βAP(Phe(SO3H)24)25-35-lesioned, nine shamoperated and eight naive control animals were used. βAP(Phe(SO3H)24)25-35-treated rats showed a significant hypoactivity in the locomotor arena throughout the four test sessions, compared to both sham-operated and naive control groups. However, the explorative behavior of sham-operated animals was, albeit non-significantly, also decreased. Detailed analysis of locomation 7 days post-injection (c) revealed a dramatic reduction in horizontal ambulation and rearing activities of βAP(Phe(SO3H)24)25-35 injected rats during the initial phase of the tests. There are obvious differences between the activity profiles of βAP(Phe(SO3H)24)25-35-treated and sham-operated or naive control animals. * P<0.05, ** P<0.01 vs. the sham-operated group, while significant differences between βAP(Phe(SO3H)24)25-35 lesioned and control animals are indicated as follows: P<0.01, P<0.05 (Kruskal–Wallis test). Data are presented as medians.
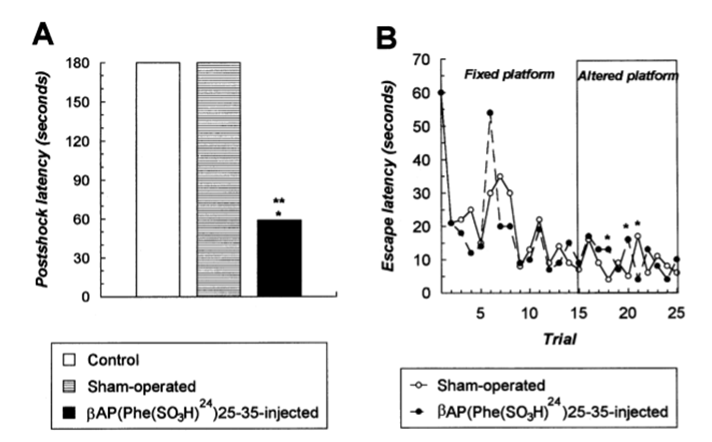 Fig. 4. Changes in post-shock latencies in the 24 h retention trial of the passive avoidance learning paradigm (a) and in spatial navigatian learning in the ‘Morris’ water maze test, using a hidden platform task (b), after βAP(Phe(SO3H)24)25-35 injections in the nbm. Rats were tested for passive avoidance learning 16 days post-operatian. βAP(Phe(SO3H)24)25-35 treatment resulted in a significant decrease in post-shock latencies indicating a marked impairment of short-term memory functions. In this experiment ten βAP(Phe(SO3H)24)25-35-lesioned, nine sham-operated and eight control animals were used in the experiment. In the water maze paradigm the time to find the platform was measured with a 60 s cut-off time over 5 daily test sessions with a 5 min inter-trial interval. Escape latencies are expressed in s. Spatial learning abilities of βAP(Phe(SO3H)24)25-35 lesioned rats did not differ from those of sham-operated animals. n=10 and n=9 for the βAP(Phe(SO3H)24)25-35 and sham-operated groups, respectively. In both experiments the results were expressed in s and are presented as medians. ** P<0.01 vs. naive controls, * P<0.05 vs. sham-operated animals (Kruskal–Wallis (a) or Mann–Whitney U (b) tests).
Fig. 4. Changes in post-shock latencies in the 24 h retention trial of the passive avoidance learning paradigm (a) and in spatial navigatian learning in the ‘Morris’ water maze test, using a hidden platform task (b), after βAP(Phe(SO3H)24)25-35 injections in the nbm. Rats were tested for passive avoidance learning 16 days post-operatian. βAP(Phe(SO3H)24)25-35 treatment resulted in a significant decrease in post-shock latencies indicating a marked impairment of short-term memory functions. In this experiment ten βAP(Phe(SO3H)24)25-35-lesioned, nine sham-operated and eight control animals were used in the experiment. In the water maze paradigm the time to find the platform was measured with a 60 s cut-off time over 5 daily test sessions with a 5 min inter-trial interval. Escape latencies are expressed in s. Spatial learning abilities of βAP(Phe(SO3H)24)25-35 lesioned rats did not differ from those of sham-operated animals. n=10 and n=9 for the βAP(Phe(SO3H)24)25-35 and sham-operated groups, respectively. In both experiments the results were expressed in s and are presented as medians. ** P<0.01 vs. naive controls, * P<0.05 vs. sham-operated animals (Kruskal–Wallis (a) or Mann–Whitney U (b) tests).
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
References
- Yamada K et al. Perspectives of Pharmacotherapy in Alzheimer’s Disease.[J]. Jpn J Pharmacol, 1999, 80(1):9-14.
- Harkany T et al. Beta-amyloid (Phe(SO3H)24)25-35 in rat nucleus basalis induces behavioral dysfunctions, impairs learning and memory and disrupts cortical cholinergic innervation.[J]. Behavioural Brain Research, 1998, 90(2):133-145.