Aβ Peptide Formation or Screening for Secretase Inhibitors
The accumulation of amyloid β (Aβ) is considered to be crucial in the pathogenesis of Alzheimer's disease (AD). Aβ deposits exist in the brains of AD patients due to an imbalanced turnover of Aβ (generation/clearance). Aβ is yielded from the amyloid precursor protein (APP) cleaved by β- and γ-secretase. Beta-site APP-cleaving enzyme 1 (BACE1) was identified to be an important β-secretase that involved in Aβ plague formation in the brain, and served as a potential biomarker for AD. Insulin-degrading enzyme (IDE) is capable of degrading soluble Aβ1-40 and Aβ1-42 into non-toxic fragments. In addition, NEP, ACE and MMP-9 are thought to be Aβ-degrading enzymes which have all been demonstrated for their capability of attenuating AD phenotypes in mice. Therefore, inhibiting the activity of either β- and γ-secretase or enhancing Aβ-degrading enzymes activity were shown to be theoretically and pharmacologically effective in the prevention of amyloid accumulation.
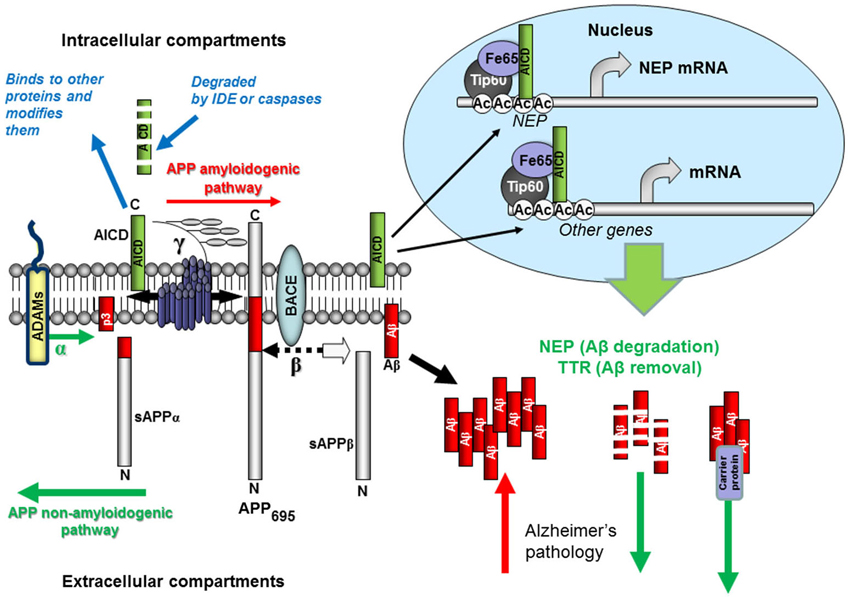 Figure 1 Schematic representation of Aβ production and removal from the brain.
Figure 1 Schematic representation of Aβ production and removal from the brain.
Creative Bioarray offers diverse customized in vitro models to help our customers identify compounds that can inhibiting secretase activity or enhancing Aβ-degrading enzymes activity against amyloid formation and accumulation. These in vitro models, including primary neuronal cultures, cell lines, iPS cells with genetic modifications, are phenotypically closer to the adult neuronal network and mimic the development of AD.
Disease Modeling and assays available
- Various cell types (both cell lines and primary neuronal cultures; human cells or other species) are available for building Alzheimer's disease models according to your application.
- APP processing assay
- beta secretase inhibitors screening
- gamma secretase inhibitors screening
- inhibitors of Aβ peptide formation screening
We provide this powerful and versatile tool for developing potential AD therapy directly as well as basic research to help our customers who wish to understand more about the underlying mechanisms of AD from which they could facilitate the process of drug development.
Study Examples
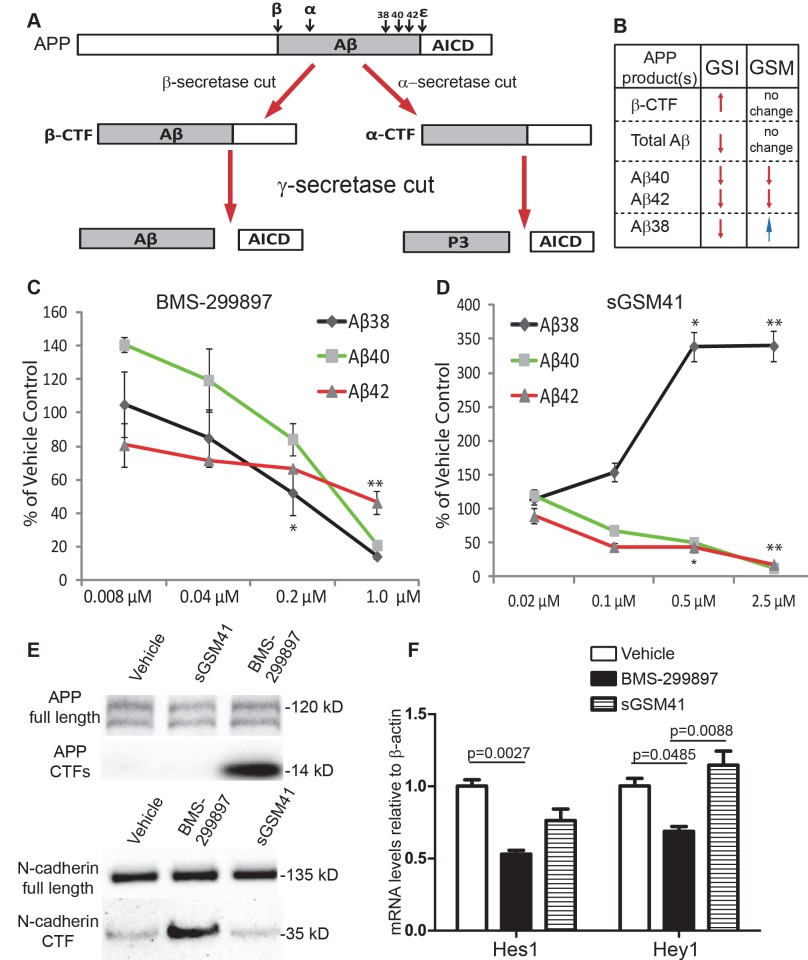 Figure 2 Differential effects of BMS-299897(γ-secretase inhibitors) and sGSM41 (γ-secretase modulators) on APP processing.
Figure 2 Differential effects of BMS-299897(γ-secretase inhibitors) and sGSM41 (γ-secretase modulators) on APP processing.
A: A diagram depicts APP processing and the pathways that GSI or GSM treatment differentially affects Aβ peptide formation and the production of APP C-terminal fragments (APP CTFs). First, β-secretase or α-secretase cleaves APP, leading to the production of either β-CTF or α-CTF. Cleavage of β-CTF by γ-secretase at multiple sites yields several Aβ peptides and the APP intracellular domain (AICD). Cleavage of α-CTF by γ-secretase gives rise to and AICD and the P3 fragment. B: Differential effects of GSI and GSM on the production of Aβ species and APP β-CTF [34–36]. Rat E18 cortical neurons (DIV7) were treated with GSI BMS-299897 (C) or sGSM41 (D) for 24 hrs. The media were harvested and levels of Aβ species (Aβ38, 40, 42) in the media were measured as described in Materials and Methods (n = 3, *P < 0.05, **P < 0.01 using student’s t-test). Treatment with 1μM BMS-299897 or 2.5μM sGSM41 showed the most robust effect and these conditions were used in all other experiments herein. E: Western blotting analyses showing the processing of two substrates of γ-secretase, APP and N-cadherin, in cortical neurons treated with vehicle (0.1% DMSO), 1μM BMS-299897, 2.5μM sGSM41 for 24 hrs. F: Quantitative measurement of mRNA levels by real-time PCR in cortical neurons treated as in E. The mRNA levels are normalized to mRNA levels of β-actin (n = 3, mean±S.E., p values represent results of Student’s t-test).
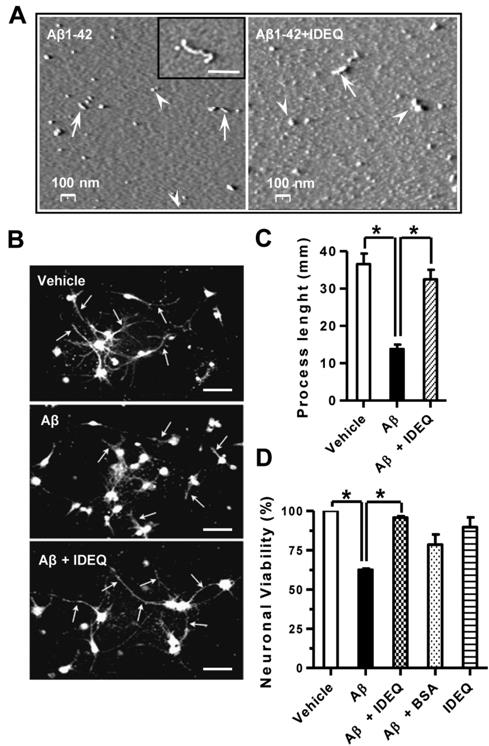 Figure 3 Aβ1-42 oligomers formed in the presence of IDEQ are not neurotoxic.
Figure 3 Aβ1-42 oligomers formed in the presence of IDEQ are not neurotoxic.
(A) Representative AFM images showing the size and morphology of Aβ1-42 neurotoxic species. Left, Aβ1-42 incubated alone for 4 days. Approximately spherical species of ∼20–30 nm are indicated by arrowheads. Rods and short protofibrils are depicted by arrows. Inset: a larger Aβ1-42 protofibril is shown. Right; Aβ1-42 incubated in the presence of IDEQ at a 1∶10 molar ratio (IDEQ: Aβ) showing larger aggregates of 50–60 nm (arrowheads) and rods with lengths of ∼100–120 nm (arrows). (B) Representative immunofluorescence of primary differentiated neurons exposed to vehicle, Aβ1-42 alone or Aβ1-42 pre-incubated with IDEQ from top to bottom, as indicated. White arrows point at neuronal processes. Bars = 30 μM. (C) Analysis of neuronal processes under the conditions as shown in panel (A). Bars represent the mean ± SEM of processes' lengths as measured from the centre of the neuronal body * p<0.01, one-way ANOVA, Tukey post-hoc test. (D) Viability of mature primary neurons after the indicated treatments as assessed by MTT reduction. * p<0.05, one-way ANOVA, Tukey post-hoc test. Results are shown for three independent experiments.
Quotation and ordering
Contact us if you have any questions. Our customer service representatives are available 24hr a day.
References
- Decourt B.; Sabbagh MN. BACE1 as a potential biomarker for Alzheimer's disease. Journal of Alzheimer's Disease. 2011, 24(s2): 53-59.
- Miners JS.; et al. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008, 18(2): 240-252.
- Nalivaeva NN.; et al. Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer's disease. Front Aging Neurosci. 2014, 6: 235.
- Weissmiller AM.; et al. A γ-secretase inhibitor, but not a γ-secretase modulator, induced defects in BDNF axonal trafficking and signaling: Evidence for a role for APP. PLOS One. 2015, 10(2): e118379.
- de Tullio MB.; et al. Proteolytically inactive insulin-degrading enzyme inhibits amyloid formation yielding non-neurotoxic abeta peptide aggregates. PLOS One. 2013, 8(4): e59113.