iPSC Characterization
- Service Details
- Workflow
- Features
- FAQ
- Explore Other Options
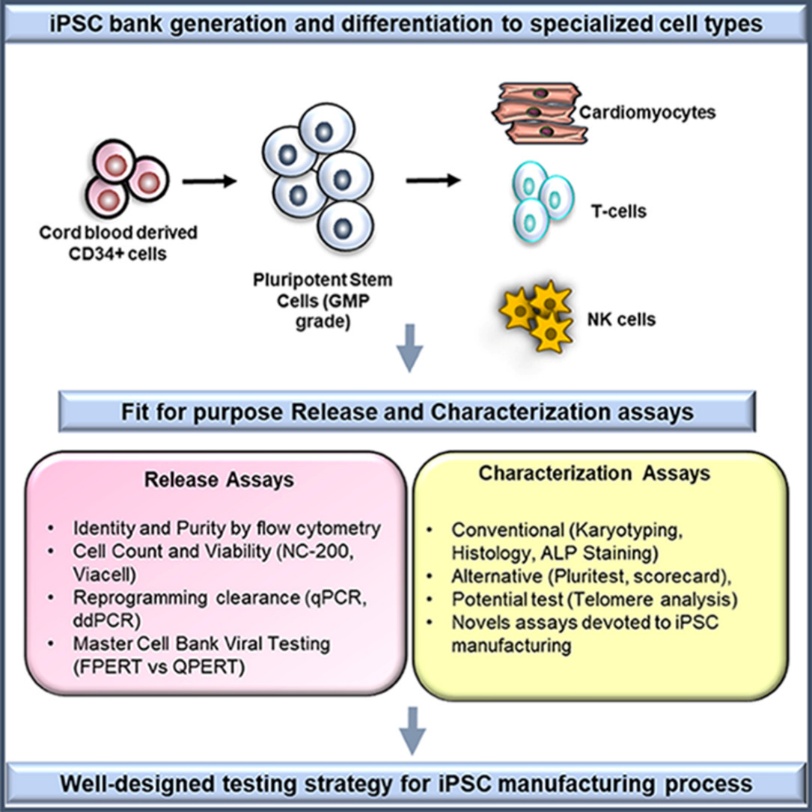 Fig. 1. Illustration of iPSC cell bank generation from CD34+ cells and characterization assays
Fig. 1. Illustration of iPSC cell bank generation from CD34+ cells and characterization assays
(Suresh Babu S, Duvvuru H, et al., 2023).
With the rapid advancement of biotechnology, the technology for generating induced pluripotent stem cells (iPSCs) has made significant progress, making it increasingly feasible to reprogram somatic cells from various sources, such as skin cells and blood cells, into pluripotent stem cells. However, a range of factors including cell source, induction method, passage number, and culture conditions influences the pluripotency, genomic stability, and differentiation potential of iPSCs. The International Society for Stem Cell Research (ISSCR) and regulatory bodies in various countries (such as the US FDA and the EU EMA) have all issued guidelines and regulations regarding stem cell research and clinical applications. These standards and regulations require comprehensive characterization of iPSCs to ensure their quality, safety, and functionality.
Creative Bioarray offers iPSC characterization services that fulfill regulatory requirements for stem cell research and clinical applications. We are committed to delivering comprehensive and accurate iPSC characterization services through advanced technology and a rigorous quality management system. Our services not only comply with international and domestic industry standards but can also be custom-designed according to the specific needs of our clients.
Creative Bioarray Offers iPSC Characterization Service as Follows (including but not limited to):
We offer comprehensive services for staining pluripotency and lineage-specific markers in stem cells. This includes live staining, immunocytochemistry, and gene expression analysis to accurately identify and quantify the pluripotent characteristics of stem cells.
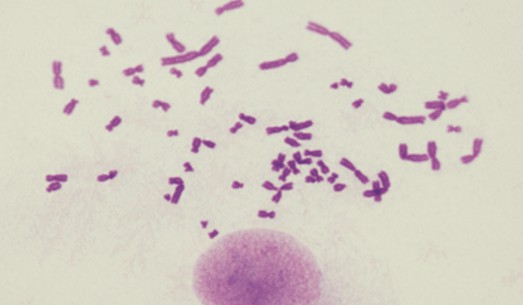
Our karyotyping services aim to detect genetic stability in cells. Utilizing advanced chromosome analysis techniques such as comparative genomic hybridization and multicolor FISH, we can identify potential genomic abnormalities or chromosomal variations.
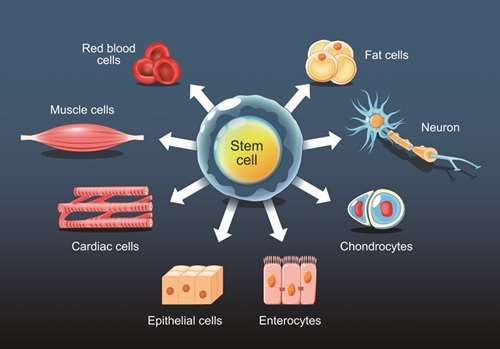
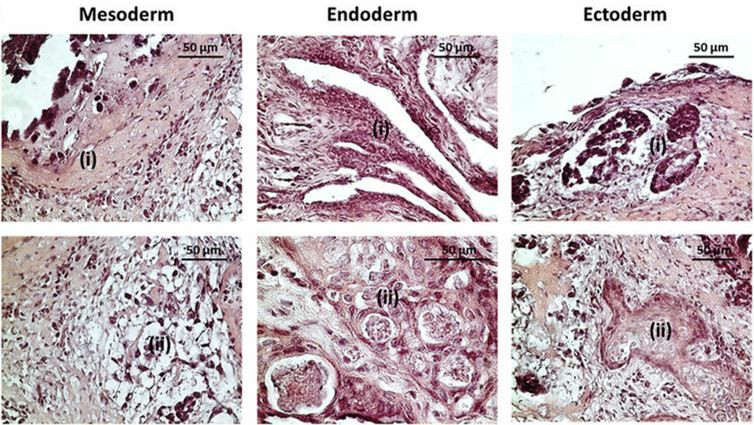
Understanding the differentiation potential of stem cells is crucial for regenerative medicine. Our services evaluate differentiation capabilities both in vitro and in vivo, including embryoid body and teratoma formation assays.
Microbiological contamination testing is vital for ensuring sterility and safety in cell cultures. We provide precise mycoplasma and other pathogen testing services to ensure a contaminant-free experimental environment.
Using DNA fingerprinting, we offer validation services for the homology of starting cells to ensure consistency across cell populations, supporting high-quality experimental results.
Our services include detecting and verifying the silencing of exogenous gene expression to ensure the efficacy and safety of transgenic applications, supporting gene therapy research.
Workflows of Induced Pluripotent Stem Cell Characterization Service
Step 1: Client Consultation and Requirement Confirmation
Engage in initial communication with the client to assess experimental needs and testing objectives. Provide relevant guidance to help select appropriate testing projects.
Step 2: Sample Reception and Preliminary Inspection
Assist in sample preparation and ensure safe receipt of samples.
Step 3: Cell Culture and Expansion
Thaw cryopreserved iPSC cells and expand cultures to obtain sufficient cell quantities for subsequent experimental identification.

Step 4: Execution of Stem Cell Characterization Experiments
Gradually perform cell characterization experiments as per client requirements, such as gene expression analysis, surface marker detection, and functional assays.
Step 5: Data Analysis and Interpretation
Collect and analyze experimental data using professional software to process the data and generate a detailed identification report.
Step 6: Report Delivery
Provide detailed experimental reports and interpret results for the client.
Why Choose Us?

Professional Expertise
Our experienced team of scientists excels in iPSC induction, culture, characterization, and application, offering extensive expertise.

High-Precision Analysis
Utilizing advanced equipment and validated analytical methods ensures highly accurate and reliable data.
Customized Solutions
We offer tailored experimental solutions based on specific client needs to meet the unique requirements of various research projects.
Rapid Delivery
Efficient service processes and project management ensure timely completion and delivery of results.
Multidimensional Characterization
Comprehensive identification services covering pluripotency marker detection, genomic stability assessment, three-germ layer differentiation capability verification, and quality testing.
FAQ
1. What is the main purpose of iPSC characterization?
The primary purpose of iPSC characterization is to confirm the pluripotency status, genetic stability, and differentiation ability of the cells. By assessing pluripotency markers, chromosomal karyotype, and three-germ layer differentiation capability, one can determine the cell's potential to differentiate into various cell types while evaluating for any genetic abnormalities or differentiation defects, ensuring reliability for subsequent research or applications.
2. Can you characterize my genetically modified cells?
Certainly. We provide characterization services for gene-edited cells. Besides routine pluripotency marker detection and chromosomal karyotype analysis, we can conduct additional tests like gene expression analysis per client requirements to ensure the characteristics and safety of the gene-edited cells.
3. Do you offer subsequent cell application development services?
Yes. In addition to iPSC characterization, we provide post-characterization cell application development services tailored to client needs, including differentiation into specific cell types (e.g., neurons, cardiomyocytes, hepatocytes), drug screening model construction, and disease model development. Our team has extensive experience in cell application development, providing clients with comprehensive, one-stop solutions.
References
- Suresh Babu S, Duvvuru H, et al. Characterization of human induced pluripotent stems cells: Current approaches, challenges, and future solutions. Biotechnol Rep (Amst). 2023. 37:e00784.
- Singh U, Quintanilla R H, et al. Novel Live Alkaline Phosphatase Substrate for Identification of Pluripotent Stem Cells. Stem Cell Rev and Rep 8, 2012. 1021–1029.
- Pino-Barrio MJ, García-García E, et al. V-myc immortalizes human neural stem cells in the absence of pluripotency-associated traits. PLoS One. 2015. 10(3):e0118499.
- Riegel M. Human molecular cytogenetics: From cells to nucleotides. Genet Mol Biol. 2014. 37(1 Suppl):194-209.
- Jiang G, Di Bernardo J, et al. Induced pluripotent stem cells from human placental chorion for perinatal tissue engineering applications. Tissue Eng Part C Methods. 2014. 20(9):731-40.
Explore Other Options