Macrophage Phagocytosis Assay
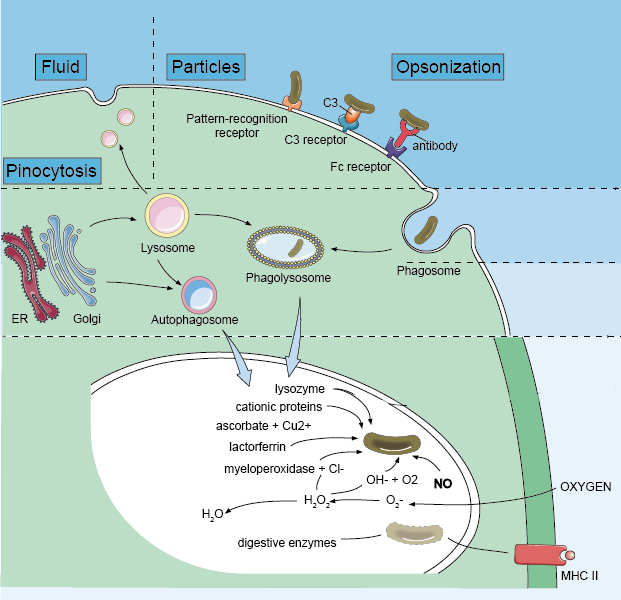
In mammals, phagocytosis of phagocytes (e.g., neutrophils, dendritic cells, and macrophages) is essential for a variety of biological events including tissue remodeling and continuous clearance of dead cells. In addition, phagocytosis is a key event that triggers host defenses against invading pathogens. Phagocytosis involves a series of events, starting with the recognition and binding of cell surface receptors to the particles, then forming an actin-rich membrane extension around the particle, and finally, the membrane extensions fuses to form phagosomes. Pathogens in the phagosomes are destroyed by lowered pH, hydrolysis, and radical attack. These early events, mediated by the innate immune system, are critical to the survival of the host. As a result of this process, pathogen-derived molecules can be presented at the cell surface (antigen presentation), allowing the induction of acquired immunity.
Reagents and Equipment
| Macrophages | GFP labelled Escherichia coli |
| Fetal Bovine Serum (FBS) | Phosphate buffered saline (PBS) |
| Ampicillin (100 mg/L) | Kanamycin (50 mg/L) |
| Dulbecco's Modified Eagle Medium (DMEM)/F12 | Luria Broth |
| 18 mm diameter glass coverslips | 10 cm cell culture plates |
| 37°C/5% CO2 incubator | 37°C shaking incubator |
| Spectrophotometer | Centrifuge |
| Glass microscope slides | Fluorescent microscope |
Macrophage Preparation
- Seed macrophage cells at 5 x 104 cells/well in a 12 well plate onto 18 mm diameter glass coverslips. Incubate overnight in a 37°C/5% CO2 incubator.
Note: Complete macrophage media including DMEM/F12, 10% FBS, and 1% appropriate selection antibiotics (e.g. if the bacteria you will be using are resistant to kanamycin and ampicillin, add those to macrophage media. Otherwise use penicillin and streptomycin).
Bacteria Preparation
- Grow up bacteria (GFP labelled E. coli) overnight before phagocytosis experiment. 100 µL stock in 100 mL Luria Broth with appropriate selection antibiotics. Grow at 37°C in a shaking incubator to an OD600 of 1.
Note: Luria Broth contains 5 g/L yeast extract + 5 g/L NaCl + 10 g/L peptone/tryptone + 1 L milli Q water. Autoclave and add antibiotics after media has cooled.
Assay Procedure
- Spin down bacterial culture for 5 min at 13,000 g. Resuspend in appropriate amount of PBS (dependent on required final concentration of bacteria and amount required for assay).
- Remove macrophage media from the wells and replace with fresh media. Supplement GFP E. coli at a ratio of 10 cells/macrophage. There should be approximately 105 macrophages and 106 bacteria per mL. Don't add bacteria to control wells.
- Co-incubate cells for 1/8/24/48 hours (or other time intervals) at 37°C/5% CO2 to allow for bacterial uptake.
- Stop phagocytosis by adding 500 µL ice-cold PBS to the well. Wash the cells three times with cold PBS.
Note: The washing steps aim to remove as much of the non-adherent bacteria as possible. Macrophages are adherent cells that should not be washed off during these washing steps. - Transfer coverslips to a microscope slide with a drop of PBS. Ensure the side with the macrophages attached is face down on the microscope slide.
- Seal microscope slides and allow to dry.
- Analyze cells using a fluorescent microscope to determine if the macrophages have phagocytosed the bacteria. Image 15 macrophages per coverslip x 4 replicates = 60 macrophages in total per treatment per time point.
- Suggested parameters to investigate: 1) percentage of macrophages containing bacteria;
2) average number of intracellular bacteria per macrophage.
References
- Yan Q. et al. Macrophage phagocytosis assay of Staphylococcus aureus by flow cytometry. Bio Protoc, 2015, 5(4): e1406.
- Lian H. et al. Microglial phagocytosis assay. Bio Protoc, 2016, 6(21): e1988.
Cell Services:
Cell Line Testing and Assays: