Generation of iPSC Lines from LQTS Patients
Stem Cell Research. 2023 Feb; 66: 103003.
Authors: Jimenez-Tellez N, Vera CD, Yildirim Z, Vicente Guevara J, Zhang T, Wu JC.
INTRODUCTION
Long QT syndrome (LQTS) is an inherited cardiovascular disorder characterized by electrical conduction abnormalities leading to arrhythmia, fainting, seizures, and an increased risk of sudden death. There are over 15 genes involved in causing LQTS, including SNTA1. Here we generated two human-induced pluripotent stem cell (iPSC) lines from two LQT patients carrying a missense mutation in SNTA1 (c.1088A > C).
METHODS
- Reprogramming. Reprogramming into iPSCs using the reprogramming kit. The cells were resuspended and plated in a Matrigel-coated plate. On day 7 post-transduction, the medium was switched to medium until day 10-15. At this stage, colonies appeared that were used for clone picking. The selected colonies were then expanded and frozen down as previously described.
- Cell culture. iPSCs were maintained in 6-well Matrigel-coated plates (1:250) under sterile conditions using the medium in a humidified incubator with 5% CO2 at 37°C. Passages were done with 0.5 mM EDTA in PBS. 10 μM of ROCK inhibitor was added to the media for the first 24 h after every passage. Media was changed every other day and monitored until confluency.
- Trilineage differentiation. The iPSCs underwent differentiation into the three germ layers (endoderm, mesoderm, and ectoderm) at p12 using the differentiation kit.
- Immunofluorescence. P11 iPSCs were fixed at 70% confluency while p12 iPSC-differentiated germ layers (endoderm, mesoderm, and ectoderm) were fixed at day 4 of trilineage differentiation. For fixation, 10% formalin was added for 15 min at room temperature. Cells were permeabilized with 50 μg/mL digitonin for 10 min and then blocked with 1% Bovine Serum Albumin and 5% serum at room temperature. Incubation with the primary antibodies was carried out overnight at 4°C. The cells were washed and incubated with the secondary antibodies for 30 min at room temperature.
- Karyotyping. Two million iPSCs were collected from each line at passage 12 and analyzed using the assay.
- Short tandem repeat analysis. To authenticate the origin of the iPSC lines, genomic DNA from PBMCs and iPSCs (p12) was isolated and purified. Afterward, the PCR amplification kit was utilized, and the amplified products were examined using capillary electrophoresis.
- Browse our recommendations
Creative Bioarray provides professional products and services, including but not limited to the following.
| Product/Service Types | Description |
| iPSC Reprogramming Kits | Creative Bioarray offers a broad range of kits and related reagents that are useful to stem cell scientists interested in performing reprogramming trials. |
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | By providing high-quality immunohistochemistry (IHC) and immunofluorescence (IF) services to customers for many years, Creative Bioarray will give you comprehensive service in regular and customized IHC and IF services. |
| Molecular Karyotyping (aCGH) Service | Creative Bioarray offers a wide range of Karyotyping Services (Traditional Karyotyping-G Banded, M-FISH, Molecular Karyotyping, etc.) to meet your different needs. |
RESULTS
- Here, we generated two iPSC lines from a 42-year-old female and a 9-year-old female. Donor cells were peripheral blood mononuclear cells (PBMCs) from individuals carrying one SNTA1 (c.1088A > C, coding for p. Glu363Ala) variant. The PBMCs from both individuals were reprogrammed into iPSCs using a Sendai virus vector containing the Yamanaka factors (Oct3/4, Sox2, Klf4, and c-Myc).
- The iPSC clones from both cell lines showed typical morphology presenting a normal karyotype. These iPSC lines showed the expression of pluripotency markers OCT3/4, NANOG, and SOX2 as detected by immunostaining and by reverse transcription-quantitative PCR for SOX2 and NANOG. Furthermore, the iPSC lines could differentiate into the three germ layers, endoderm, mesoderm, and ectoderm. Short tandem repeat (STR) analysis proved that the genetic origin of these iPSC lines was the same as that of their donor PBMCs.
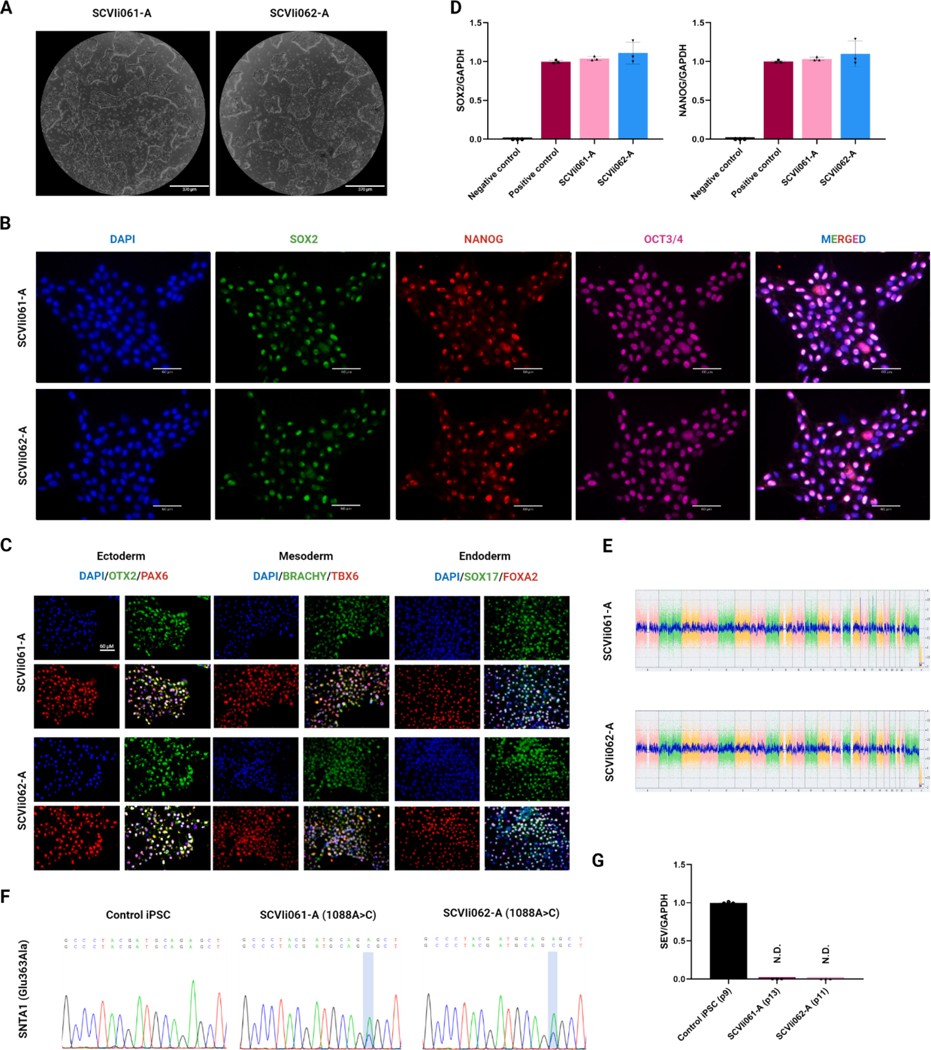 Fig. 1 Characterization of iPSC lines derived from two long QT patients carrying an SNTA1 mutation (c. 1088A > C).
Fig. 1 Characterization of iPSC lines derived from two long QT patients carrying an SNTA1 mutation (c. 1088A > C).
SUMMARY
Patients carrying the SNTA1 mutation (c.1088A > C) showed a long QT phenotype. The iPSC lines generated from these patients will provide a source for cardiomyocyte differentiation under in vitro conditions which can be used to study the pathophysiology of the disease, serving as a potential tool for drug screening.
RELATED PRODUCTS & SERVICES
Reference
- Jimenez-Tellez N, et al. (2023). "Generation of two iPSC lines from long QT syndrome patients carrying SNTA1 variants." Stem Cell Res. 66, 103003.