Electroporation of hiPSCs to Deliver CRISPR-Cas9 Plasmids
Human induced pluripotent stem cells (hiPSCs) are the ideal cell source for autologous cell replacement. CRISPR-based genome editing of patient-specific iPSCs shows great promise for future autologous cell replacement therapies. One caveat, however, is that hiPSCs are notoriously difficult to transfect, and optimized experimental design considerations are often necessary.
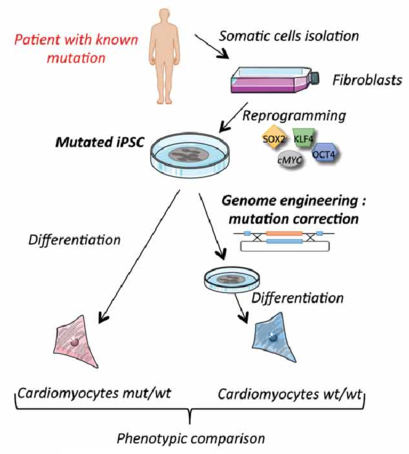 Figure 1. Mutation correction strategy of patient fibroblasts.
Figure 1. Mutation correction strategy of patient fibroblasts.
Materials and Equipment
| Recombinant human laminin-521 | Dulbecco’s phosphate-buffered saline (DPBS) |
| Complete E8 medium (Essential 8 medium, 10 ng/mL rhFGF2, 100 μg/mL Primocin) | Homology-directed repair plasmid (pHDR, optional) |
| 100X RevitaCell Supplement | NEON Transfection System |
| pX330 (CRISPR-Cas9 plasmid) | Versene solution |
| Sterile 6-well tissue culture plates | Sterile microcentrifuge tubes/conical tubes |
| 37°C, 5% CO2 incubator | Cell counter or hemocytometer |
Prepare hiPSCs for Transfection
1) Dilute 1 mL rhLaminin-521 per 19 mL DPBS and coat each well using 1.5 mL solution. Incubate 2 h at 37°C or seal plates and incubate overnight at 4°C.
Note: a) This step requires advanced planning for the number of plates required and the extended coating times. b) To reduce the scale and cost of reagents, one can coat less than six wells of a given plate. c) Coated plates are stable for 1 week at 4°C.
2) Expand hiPSCs in complete E8 medium on laminin-coated 6-well plates.
3) To begin electroporation, pretreat hiPSCs with 1.5 mL/well of E8 medium containing 10 ng/mL rhFGF2 and 1X RevitaCell for 30 min in the 37°C incubator.
Note: a) Because electroporation is harsh on cells, removal of antibiotics (Primocin) is recommended by the manufacturer to reduce cell stress after treatment. b) RevitaCell contains a ROCK inhibitor, which may result in a spindle-shaped cell morphology. This change is temporary and will not affect the pluripotency of the line. The morphology will reverse after RevitaCell is removed.
4) Aliquot the appropriate amount of plasmid into a 1.5 mL microcentrifuge tube. For gene disruption, use 2 μg pX330 per reaction. For gene correction, use 1.2 μg pX330 and 800 ng pHDR per reaction.
Note: a) The amounts of pX330 and pHDR assume a 1:1 molar ratio of a 10 Kb pX330 and 6 Kb pHDR. The volume of plasmid DNA should not exceed 10% of the electroporation reaction. b) The total amount of plasmid DNA can be increased, but cell viability will decrease.
5) Aspirate medium from pretreated cells and incubate with 1.5 mL/well Versene solution for 3-5 min at 37°C to loosen cellular attachments.
6) Aspirate Versene, then gently lift the cells with 1 mL DPBS to form a single cell suspension and transfer the suspension to a sterile conical tube. Take care to avoid over-pipetting the cells.
Note: hiPSCs are viable in DPBS for at least an hour. For experiments spanning multiple hours, cells can be transfected in batches.
7) Count cells using a cell counter or hemocytometer and determine the total amount of suspension needed to give 7-10 x 105 cells/transfection, including a plasmid-free control. Transfer this volume to a new sterile conical tube.
Perform Electroporation
1) Centrifuge cells 3 min at 100 g, room temperature.
2) During centrifugation, remove buffer from the required number of laminin-coated plates, and replace with 1.5 mL/well fresh E8 medium containing 10 ng/mL rhFGF2 and 1X RevitaCell.
3) Aspirate supernatant from the cells without disturbing the pellet. Gently resuspend pellet in 120 μL/reaction buffer and transfer the suspension to the appropriate plasmid-containing microcentrifuge tube(s), including one tube for the control.
4) Program the NEON Transfection System using the following parameters: 1100 V, 30 ms and 1 pulse.
5) Fill the NEON Tube with 3 mL Buffer E2 and insert the tube into the NEON Pipette Station as manufacturer’s instructions.
6) Aspirate the cell/plasmid mixture from the first tube using the NEON Pipette and a 100 μL tip.
Note: Avoid air bubbles in the tip, as this will decrease the electroporation efficiency.
7) Place the Neon Pipette vertically into the Neon Tube within the Neon Pipette Station and operate the system according to the manufacturer’s instructions.
8) When the protocol finishes, remove the Neon Pipette and transfer the treated cells to the appropriate well of a plate from step 2).
9) Repeat until all transfections are complete, then incubate plates overnight at 37°C, 5% CO2.
10) Replace medium with complete E8 medium containing 1X RevitaCell. Return to incubator for 24 h before proceeding to any downstream protocols.
Note: Plasmid-based transfections require time for nuclear incorporation and expression.
Positive Selection of Genomically Modified hiPSCs
1) Aspirate medium from genomically modified cells and replace with 2 mL complete E8 medium containing 0.05 μg/mL puromycin for 24-48 h.
Note: For wells that are >70% confluent, passage cells 1:6 before beginning selection.
2) Treat cells with 2 mL complete E8 containing 0.1 μg/mL puromycin for 1-5 days.
3) Treat cells with 2 mL complete E8 containing 0.5 μg/mL puromycin for another 1-4 days.
Note: a) Cells in the untreated control should be nearly or completely dead. b) Surviving colonies in treated wells should be evident at this point, and large enough to passage for expansion and further analysis.
4) Passage positively selected clones.
Note: a) Drug selection is excluded during passaging. b) Care must be taken when loosening a colony, as applying too much pressure will dislodge the colony. If this occurs, change the medium to avoid contaminating subsequent colonies.
5) Treat isolated colonies with complete E8 medium containing 0.5 μg/mL puromycin.
Note: Not all of the isolated clones survive at this point, which is normal.
6) Expand surviving clones on laminin-coated 6-well plates. Maintain at least three wells per clone, so that gDNA and RNA can be collected and analyzed.
References
- Giacalone, J. C. et al. CRISPR-Cas9-based genome editing of human induced pluripotent stem cells. Current Protocols in Stem Cell Biology, 2018, 44: 5B.7.1-5B.7.22.
- Somali Chaterji, et al. CRISPR genome engineering for human pluripotent stem cell research. Theranostics, 2017, 7(18): 4445-4469.
- Bassett A. R. et al. Editing the genome of hiPSC with CRISPR/Cas9: disease models. Mamm Genome, 2017, 28(7): 348-364.