DNA Laddering Assay
One of the distinctive biochemical features of apoptosis is the cleavage of DNA by a specific nuclease called caspase-activated DNase (CAD). During apoptosis, the DNA strand is cleaved by CAD and generates a number of DNA fragmentations of 180-200 base pairs known as DNA ladders. These DNA fragments can be extracted from the cells and visualized using agarose gel electrophoresis. Agarose gel electrophoresis is a simple technique but generally able to provide robust results. In order to compare the degree of apoptosis between experimental samples, more quantitative methods need to be combined.
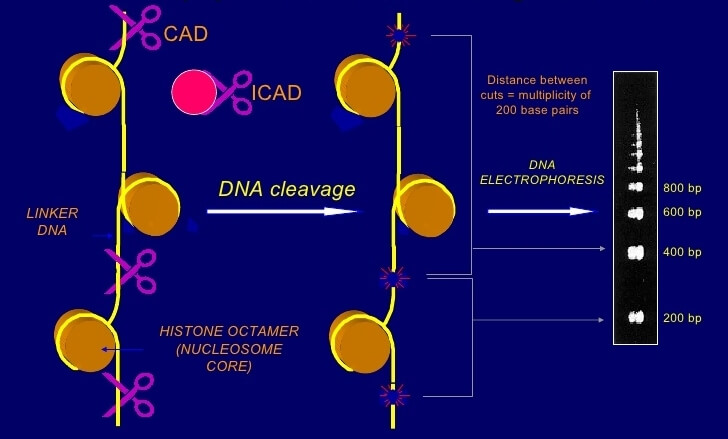 Figure 1. Apoptosis-induce DNA fragmentation.
Figure 1. Apoptosis-induce DNA fragmentation.
Materials
- TAE
- Agarose
- Gel loading buffer
- DNA size markers
- Ethidium bromide
- Proteinase K
- RNase cocktail
- TES lysis buffer
Equipment
- Standard gel electrophoresis equipment
- Refrigerated cell centrifuge
- UV transilluminator
- DC power supply
- Heating block
- Microfuge
Assay Procedure
- Prepare 0.5 mL of cell suspension (The number of cells is no less than 5 x 105 and no more than 5 x 106).
Note: No less than 5 x 105, otherwise DNA will not be detected, and no more than 5 x 106, to avoid difficult handling of insoluble DNA. - Centrifuge cells at 2000 rpm at 4°C for 10 min.
- Add 0.5 mL of TES lysis buffer and vortex vigorously.
Note: This procedure allows the release of fragmented chromatin from nuclei, after cell lysis and disruption of the nuclear structure. - Add 20 µL of RNase cocktail and mix well by flipping the tip of the tube.
- Incubate for 30-120 minutes at 37°C.
- Add 20 µL of proteinase K and mix by flipping the tip of the tube.
- Incubate at 50°C for at least 90 minutes or, if preferred, overnight.
- Mix the samples of DNA with loading buffer.
Note: The addition of loading buffer to samples allows to load gel wells more easily and to monitor the run of samples. - Load 10-20 µL of DNA samples to each well of a standard 1% agarose gel containing 0.5 µg/mL ethidium bromide.
Note: Ethidium bromide is a potential carcinogen, please wear gloves and handle with care.
Instead of directly adding ethidium bromide into the agarose gel, DNA can also be visualized by staining the gel after electrophoresis in 1 µg/mL ethidium bromide (containing TAE buffer) for 1 hour. - Run the gel at a low voltage, which improves resolution of DNA fragments (35 V for 4 hours or until loading dye has run two-thirds of the way down the gel).
Note: During electrophoresis, it is possible to monitor the migration of samples by following the migration of bromophenol blue dye contained in the loading buffer. - Visualize the DNA by a UV light source and documented by photography.
Note: Wear eye and skin protection when UV are on.
Apoptotic cells can form a distinct DNA ladder, while necrotic samples may generate a smear pattern (or no DNA splitting). DNA from viable cells will remain at the top of the gel as a high-molecular-weight band.
References
- Bortner, C.D. et al.; The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995, 5: 21.
- Duke, R.C. et al.; Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc. Natl. Acad. Sci. U.S.A. 1986, 80: 6361.
Cell Services:
Cell Line Testing and Assays: