Detection of Phosphorylated MAPK by Immunocytochemistry
Here we describe an immunocytochemistry (ICC) method for staining against phosphorylated forms of the mitogen-activated protein kinases (pMAPK). Phosphorylation is induced by a number of stimuli such as insulin and epidermal growth factor (EGF), and is a prerequisite for many cellular processes including cell proliferation and survival. ICC allows cell-type specific and subcellular monitoring of kinase activation by using antibodies raised against specific phosphorylation sites.
This protocol uses fluorescence-labeled antibody reagents to allow staining of tissue culture cells, blood cells, thin tissue sections, or lower organisms. It involves fixation of the cells or tissues to be examined, brief permeabilization of the plasma membranes to allow immunological recognition of intracellular components, and fluorescent detection. This method is especially useful to determine localization of signaling events, but is less accurate in determining the level of activations.
Materials
HEK293 cells
Insulin
Hoechst
Phosstop
Triton X-100
Sodium azide
2.5% Trypsin and 0.2 M EDTA
DMEM culture medium (10% FBS)
4% Paraformaldehyde (PFA) in PBS
Primary antibody (anti-phospho-MAPK)
Secondary antibody (FITC-conjugated)
Equipment
Coverslip
6-well plate
Incubator
Confocal or fluorescent microscope
Cell Preparation
- The HEK293 cells are maintained in DMEM culture medium, until they reach a confluence of 100%.
- At 100% confluence, the cells are dissociated from the culture well by removing the cell medium and adding 0.25% trypsin and 0.2 M EDTA.
- Adding DMEM culture medium to resuspend the cells.
- Placing a 22 mm2 coverslip in each well of a 6-well tissue culture plate.
- The resuspended cells are seeded at a density of 30,000 in each well and maintained at 5% CO2 and 37°C until reaching a density of 70%.
Stimulation and Fixation of Cultured HEK293 Cells
- The medium is removed and replaced with 0.5 mL DMEM medium containing 10 nM insulin. At the same time, HEK293 cells are stimulated with media containing sterile PBS as control. The cultures are then incubated for 10 minutes at 37°C in a humidified incubator with 5% CO2.
- After 10 minutes of incubation, the cells are placed directly on ice to decrease the dephosphorylation rate catalyzed by phosphatases. The medium is removed and the cells are washed in ice-cold PBS before adding 0.5 mL of ice-cold 4% PFA containing phosstop to prevent dephosphorylation during fixation.
Note: It is critical to prevent dephosphorylation of phosphorylated proteins during the fixation step in order to be able to detect phosphorylation by immunostaining. - The cells are fixed for 20 minutes on ice before three times wash in PBS. The last washing step includes adding 0.01% sodium azide to prevent bacterial contamination, and the fixed cells are hereafter stored at 4°C until use.
Immunocytochemistry of HEK293 Cells
- The fixed HEK293 cells are washed briefly in PBS to remove the 0.01% sodium azide.
- The cells are permeabilized by washing 3 x 5 minutes in PBS containing 0.1% Triton X-100, at RT.
Note: Other methods of fixation can be used, but paraformaldehyde fixation followed by permeabilization with 0.1% Triton X-100 is a very effective method when antibodies directed against signaling components are used. - After this, the coverslips are washed once in PBS.
- Unspecific binding of the antibodies is reduced by incubating the cells with 0.5 mL PBS containing 10% FBS (blocking buffer) for 30 minutes at RT.
- After this, the cells are incubated with primary antibody (anti-phospho-MAPK), diluted in blocking buffer, left at 4°C overnight in a humidified chamber.
- Before continuing the next morning, the coverslips are placed at RT for 1 hour to enhance antibody-antigen binding.
- To remove the primary antibody, the coverslips are washed 3 x 5 minutes in PBS containing 0.1% Triton X-100.
- The cells are then incubated with FITC-conjugated secondary antibody, diluted 1:300 in blocking buffer, in a dark humidified chamber at RT for 4 hours.
- The cells are then washed 3 x 5 minutes in PBS, and 5 μg/mL Hoechst nuclear staining is included in the last wash.
- The coverslips are mounted using fluorescence mounting medium and stored at 4°C.
- Finally, the immunostainings are monitored with either a normal of confocal fluorescent microscope.
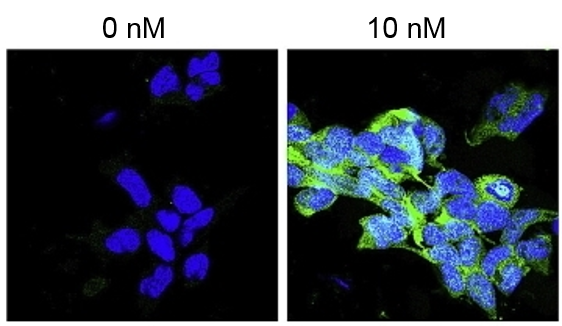 Figure 1. Immunocytochemical staining of pMAPK in HEK293 cells
Figure 1. Immunocytochemical staining of pMAPK in HEK293 cells
References
- Zhong Y, et al.; Immunological detection of phosphorylation. Current Protocol in Cell Biology, 2001, 14.2.1-14.2.15.
- Molgaard S, et al.; Detection of phosphorylated Akt and MAPK in cell culture assays. MethodsX, 2016, 3: 386-398.
Cell Services
Cell Line Testing and Assays