Calcein AM Cell Viability Assay
Introduction
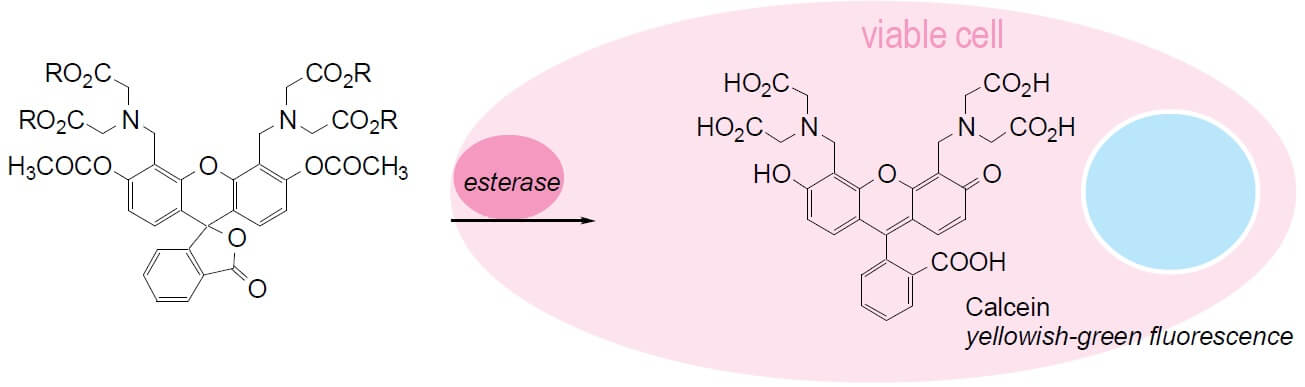
Calcein AM is a widely used membrane-permeable cell marker that can permeate into intact cells. Once inside the cells, non-fluorescent calcein AM is hydrolyzed by intracellular esterases into the green fluorescent dye calcein, which is highly negatively charged and is well-retained in cell cytoplasm. Calcein AM has been used for studies of cell membrane integrity and for long-term cell tracking due to its low cellular toxicity. It has also been used to quantify the number of viable cells.
The calcein AM cell viability assay is an endpoint analysis method for cell viability. The fluorescent signal generated from the assay is proportional to the number of living cells in the sample. And the results can be obtained quickly, mostly within 2 hours.
Key Features
- Ideal for both suspension and adherent cells
- Rapid and high-throughput
- Easy to use
- High sensitive
- Better retention and brightness compared to other fluorescent compounds (e.g. fluorescein)
- Adaptable to a wide variety of techniques (microplate, immunocytochemistry, flow cytometry)
Applications
- Measurement of cell proliferation in response to growth factors, cytokines, mitogens and nutrients.
- Analysis of cytotoxic and cytostatic compounds such as anticancer drugs, toxic agents and other pharmaceuticals.
- Assessment of physiological mediators and antibodies that affect cell growth.
Materials and Equipment Required
- Calcein AM
- Calcein AM buffer
- Centrifuge
- Culture microplates
- Fluorescence plate reader
- Deionized or distilled water
- Cell culture medium
- CO2 incubator
- DMSO
Reagent Preparation
- 1X Calcein AM DW Buffer - Dilute the Calcein AM DW Buffer to 1X according to your instruction manual before use.
- Calcein AM - Dissolve 50 μg of Calcein AM in 25 μL of anhydrous DMSO to make a 2 mM Calcein AM Stock Solution.
- Immediately prior to use, dilute the Calcein AM Stock Solution in 1X Calcein AM DW Buffer to a Calcein AM Working Solution, preparing enough for all wells using 50 μL/well at the appropriate concentration.
Note: The optimal concentration of calcein AM may vary depending on cell type. In general it is best to use the lowest dye concentration that gives sufficient signal.
Assay Protocol
- Plate cells in a microplate with the densities from 1,000 to 500,000 cells/mL. The range of cell concentrations will need to be optimized to ensure the best dynamic range.
- Centrifuge at 250 x g for 5 minutes. Alternatively, transfer the cells to microfuge tubes for centrifugation and return to the plate to read.
- Carefully discard the media supernate and add 100 μL of 1X Calcein AM DW Buffer.
- Centrifuge at 250 x g for 5 minutes.
- Remove the supernate and replace with 100 μL of fresh 1X Calcein AM DW Buffer.
Note: It is important to remove any carry-over media in the supernate, as phenol red and serum will interfere with the sensitivity of the assay. - Add 50 μL of freshly diluted Calcein AM Working Solution to each well.
- Incubate for 30 minutes at 37°C.
- Record fluorescence using a 490 nm excitation filter and a 520 emission filter.
Troubleshooting Guide
| Problems | Actions |
| Low fluorescence values | Increase the concentration of Calcein AM. Check the health of the cells (Trypan Blue, etc.). Incubate the plate in the dark. |
| High background | Use black-walled microplates. Use freshly diluted Calcein AM. Increase the washing times to ensure medium removal. Shorten the incubation time with Calcein AM. Decrease the number of cells. |
| Poor repeatability | Make sure no bubbles in the well. Check the accuracy of the pipette. Ensure that there are no cells loss during wash steps. |
Reference
- Wang X.M. et al.; A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol, 1993, 37: 264.
Cell Services:
Cell Line Testing and Assays: