Culture of Primary Mouse Embryonic Fibroblasts
Introduction
Mouse Embryonic Fibroblasts (MEFs) are a type of fibroblast derived from the mouse embryo. MEFs have a typical characterization of spindle shape in vitro which is a typical feature of fibroblasts. As a kind of primary cell, MEFs will senescence and finally die off after limited transmission. Nevertheless, several strategies like virus infection or repeated transmission are used to immortalize MEF cells, which is favored for conducting a research. MEFs are widely used in life science researches, especially in stem cell biology and oncology.
At Creative Bioarray, in addition to commercial established MEFs and related culture materials, custom primary cell culture services are also provided. If you have any demand, please feel free to contact us.
Here is a protocol of MEFs' culture.
Materials and Reagents:
- Dulbecco's Ca2+ - and Mg2+- free PBS (D-PBS)
- 0.05% trypsin
- 0.53 mmol/L EDTA solution
- MEF culture medium (High-glucose DMEM; 10% FBS; 1X penicillin-streptomycin solution)
- Freezing media: 10% DMSO and 90% FBS
Equipment
- Sterile plastic serological pipettes, 5 and 10 ml
- Sterile disposable tubes (15 and 50 ml)
- T75 tissue culture flasks
- 37 °C 5% CO2 tissue culture incubator
- Inverted microscope
Methods
• Subculture of MEF
Mouse embryonic fibroblasts should be subcultured when they reach 80–90% confluence. If the MEF are allowed to reach 100% confluence, growth arrest can result in a decrease in the subsequent proliferative potential of the cells.
1. Aspirate the MEF medium and wash the monolayer of cells with 2–3 mL of room-temperature D-PBS to remove any residual growth medium.
2. Aspirate the D-PBS and add 3–4 mL of room-temperature trypsin–EDTA (T/E). Incubate the dishes at 37°C for 3–5 min.
3. Once the cells have begun to detach, transfer them to a centrifugation tube containing 6–7 mL MEF medium (which contains sufficient FBS to inhibit the trypsin activity) for centrifugation. Residual cells can be collected by rinsing the dish once or twice with 5 mL of the cell/medium mixture.
4. Centrifuge at 1200 rpm (300g) for 5–7 min.
5. Aspirate the T/E containing medium and add fresh MEF culture medium (3 mL per initial input dish).
6. Split the cells at a 1:3~1:5 ratio to expand their numbers. Labeled as "P2" to indicate that this is the second plating of these cells.
7. After 2–3 d, the cells should again reach confluence and are ready to use or to cryopreserve.
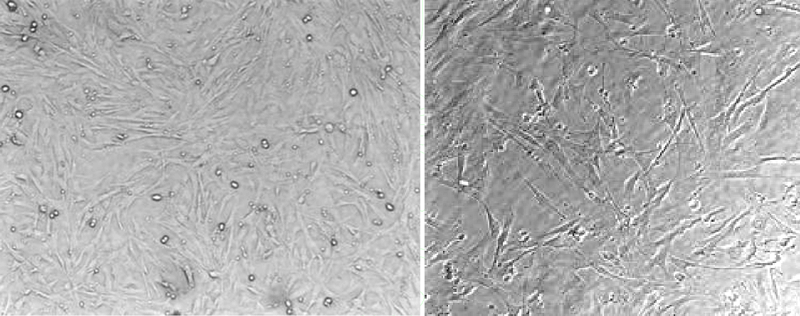 Mouse embryonic fibroblasts (100x)
Mouse embryonic fibroblasts (100x)
NOTES
1. Avoid cells contamination.
2. Short digestion time.
• Cryopreserved and Thawing of MEF
1. Cryopreserved
1.1 Prepare cryoprotective agents (CPAs) for a total volume of 1.5 ml, 10% DMSO and 90% FBS.
1.2 Harvest MEF cells by room-temperature trypsin–EDTA (T/E) from culture flasks and centrifuging in new DMEM media.
1.3 Dissolve cell pellet in CPASs and transfer cell suspension in cryovials immediately. After record the date and name, put the cryovials into a propanol-based freezing device.
1.4 Finally, transfer cryovials into liquid nitrogen and record the position of samples in register or computer.
2. Thawing
2.1 Put cryovials in 37°C water bath to thaw the cells.
2.2 Add 5~10ml MEF culture medium to resuspend cells. Centrifuge at 1200 rpm (300g) for 5–7 min in order to remove DMSO.
2.3 Resuspend cells by MEF culture medium. And transfer the cell suspension to flasks or plates by using a sterile pipette.
2.4 Allow to grow them in CO2 incubator.
NOTES
- Cell numbers of cryopreserved should be more than 106 cell/ tube.
- Don't directly put the cryovials into liquid nitrogen.
- When thawing, just stop at a small ice-clump remaining. Minimizing the thawing time as much as possible to keep high yield of viable cells.
- Keep sterile through the whole process.
References
- Freshney, R. I. Culture of Animal Cells. A Manual of Basic Techniques, 2nd ed. 2000.
- Celis, J. F. Cell Biology: A Laboratory Handbook, 2nd ed. 1998.