7-AAD/CFSE Cell-Mediated Cytotoxicity Assay
Cell-mediated cytotoxicity is characterized by the recognition and destruction of target cells (tumor or intracellular pathogen infected cells) by effector cells such as cytotoxic T lymphocytes (CTL) and natural killer (NK) cells. This process is mediated by antibody-dependent cell-mediated cytotoxicity (ADCC), lymphocyte-mediated cytotoxicity, and complement-mediated cytotoxicity. Evaluation of these pathways is one of the most important immunoassays for monitoring the status of the immune system.
7-AAD/CFSE cell-mediated cytotoxicity assay employs a green fluorescent probe CFSE (carboxyfluorosuccinimide ester) to label live target cells and a red fluorescent probe 7-AAD (7-aminoactinomycin D) to label late apoptotic or necrotic target cells. CFSE passively diffuses into the cells and its acetate groups are cleaved by intracellular esterases to produce highly fluorescent carboxyfluorescein succinimidyl ester. 7-AAD is a membrane-impermeable dye that is generally excluded from living cells but penetrate dead or damaged cells to label DNA.
This method does not require lysis of cells and can directly measure cytotoxicity. In addition, the flow cytometry-based method provides robust data and supports multi-parametric analysis.
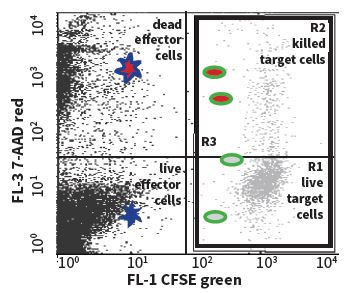 Figure 1. The illustration of staining target cell populations.
Figure 1. The illustration of staining target cell populations.
Applications
- Measurement of cell cytotoxicity in response to drug or toxin treatment
- Quantification of the cytotoxic effect of immune effector cells on target cells
- Assessment of physiological mediators and antibodies that affect cell cytotoxicity
Materials
- 7-AAD viability dye
- CFSE stock solution
- Cell-based assay buffer
- Effector and target cells
- Appropriate culture medium
- 6-, 12-, or 24-well plate
- Flow cytometer
Reagent Preparation
- Cell-based assay buffer preparation
Dissolve the cell-based assay buffer tablet with 100 mL of distilled water and mix well to ensure that the tablet dissolves completely. The diluted assay buffer is stable at room temperature for one year.
- 7-AAD staining solution preparation
Dilute the 7-AAD viability dye according to the instruction and mix well.
- CFSE staining solution preparation
First, prepare a 0.1% BSA/assay buffer by adding 10 mg BSA to 10 mL of assay buffer. Then dilute CFSE stock solution in 0.1% BSA/assay buffer and mix well.
Setting the Control
To properly analyze the data, the following control target cell groups are needed to set up the flow cytometer and compensation.
- Unstained target cells (without CFSE and 7-AAD staining)
- Single-stain target cells (with CFSE staining only or 7-AAD staining only)
Labeling the Target Cells
- Seed the target cells in a 6-, 12-, or 24-well plate at a density of 105-106 cells/well in 2, 1, or 0.5 mL of culture medium, respectively.
- Culture the cells in a CO2 incubator at 37°C for at least 24 hours before treatment.
- Collect the cells and centrifuge at 400 g for 5 minutes to pellet the cells and wash once with 1 mL of assay buffer.
- Resuspend cell pellets in CFSE staining solution at a concentration of 106 cells/mL. Control target cells (target cells without CFSE) should be resuspended in 0.1% BSA/assay buffer.
- Incubate for 15 minutes at room temperature.
- Centrifuge the target cells at 400 g for 5 minutes and aspirate the supernatant.
- Resuspend the target cells in the culture medium at a concentration of 106 cells/mL.
- Centrifuge the target cells at 400 g for 5 minutes and aspirate the supernatant.
- Resuspend the target cells in culture medium at a concentration of 105 cells/mL.
- Incubate the cells in a CO2 incubator for 30 minutes or longer (but not long enough for cell proliferation) at 37°C.
Assay Procedure
- Collect effector cells and centrifuge at 400 g for 5 minutes.
- Resuspend the cells in culture medium at a concentration of 5 x 106 cells/mL.
- Add effector cells to the CFSE-labeled target cell suspension at a predetermined effector/target cell ratio. Some examples are shown in the table below:
- Incubate the cell mixture for a period of time according to your optimal protocol, allowing enough time for the progress of cytolytic activity.
- Centrifuge the cell mixture and aspirate the supernatant.
- Resuspend the cells in 0.5-1 mL of 7-AAD staining solution and mix well. The control target cells (target cells without CFSE and 7-AAD staining or target cells with CFSE staining only) should be resuspended in 0.5-1.0 mL of assay buffer.
- Incubate the cells for 15 minutes in the dark at 4°C.
- Centrifuge the cell mixture and resuspend in 0.5-1 mL of assay buffer.
- The cells are now ready for analysis by a flow cytometer and should be analyzed immediately.
Table 1. Addition of effector cells to target cell suspension.
| Effector/target cell ratio | Effector cell suspension | Target cell suspension | Target cell medium | Final volume |
| 0 | 0 mL | 1.5 mL | 0 mL | 1.5 mL |
| 6.25:1 | 0.125 mL | 1 mL | 0.375 mL | 1.5 mL |
| 12.5:1 | 0.25 mL | 1 mL | 0.25 mL | 1.5 mL |
| 25:1 | 0.5 mL | 1 mL | 0 mL | 1.5 mL |
Troubleshooting
| Problems | Possible causes | Recommended solutions |
| Low signal of CFSE | Cells are not healthy | Use only healthy cells |
| Cells are not well labeled by CFSE | Perform a titration of CFSE to get an optimal concentration of CFSE staining | |
| No difference in cytotoxicity among different effector cell to target cell ratios | Target cells are not healthy | Use only healthy target cells |
| Effector cells do not cause cytotoxicity | Use effector cells that cause cytotoxicity |
References
- Russell J. H. et al.; Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol, 2002, 20: 323-370.
- Benczur M. et al.; Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin. Exp. Immunol, 1980, 39: 657-662.
Cell Services:
Cell Line Testing and Assays: