Senescent Cells Perturb Intestinal Stem Cell Differentiation Through Ptk7
Nature Communications. 2023 Jan 11; 14(1): 156
Authors: Yun J, Hansen S, Morris O, Madden DT, Libeu CP, Kumar AJ, Wehrfritz C, Nile AH, Zhang Y, Zhou L, Liang Y, Modrusan Z, Chen MB, Overall CC, Garfield D, Campisi J, Schilling B, Hannoush RN, Jasper H.
INTRODUCTION
- Cellular senescence and the senescence-associated secretory phenotype (SASP) are implicated in aging and age-related disease, and SASP-related inflammation is thought to contribute to tissue dysfunction in aging and diseased animals. However, whether and how SASP factors influence the regenerative capacity of tissues remains unclear. Organoid systems provide an efficient and convenient model of tissue regeneration to dissect the effects of SASP components on stem cell function.
- Ptk7, also called colon cancer Kinase 4 (CCK4), is a member of the receptor tyrosine kinase (RTK) family, and contains N-terminal Immunoglobulin-like repeats (Ig), a transmembrane region, and an inactive tyrosine kinase domain. Ptk7 influences cellular processes such as migration and planar cell polarity (PCP).
METHODS
- To ask whether and how SASP factors can influence intestinal stem cell (ISC) function and regeneration, we exposed mouse intestinal organoids derived from isolated crypts of the small intestine (jejunum) to conditioned media (CM) from mouse embryonic fibroblasts (MEFs) that were induced to senesce by X-ray irradiation or doxorubicin treatment. As a control, we used CM from quiescent (serum deprived) MEFs, which were growth arrested, similar to senescent cells.
- To identify factor(s) in SCM responsible for the morphological changes in organoids, we analyzed proteins in SCM by mass spectrometry (MS). SCM from a large-scale culture of senescent MEFs was dialyzed, concentrated and fractionated by ion exchange and size exclusion columns.
- To test whether sPtk7 acts through the Wnt signaling pathway, we used inhibitory peptides. In addition to the FZD7-specific peptide inhibitor, we identified peptides binding to the cysteine-rich domains of FZD5 and FZD8 from naïve peptide libraries by phage display. To further examine the effect of Wnt signaling on organoids, we added Wnt3a and Wnt5a recombinant proteins to crypt cultures.
- Intestinal organoids were cultured in complete media, QCM or SCM (with or without anti-Ptk7), and complete media with Wnt5a (with or without anti-Ptk7) for 2 days, and subjected to RNAseq analysis. Then, we carried out whole mount immunostaining of organoids for YAP.
RESULTS
- Organoids from fresh crypts acquired a distinct spherical cyst morphology after exposure to senescent CM (SCM), while organoids exposed to quiescent CM (QCM) exhibited normal crypt budding. Whole mount immunostaining confirmed these findings, secretory cells such as goblet, enteroendocrine and Tuft cells (Dclk), and Olfm4+SCs were reduced, while other cell types, including enterocytes, were not. Critically, the morphological change caused by SASP factors was reversible. Together, these data indicated that SCM causes specific and reversible defects in cell differentiation in the intestinal stem cell lineage.
- One of the proteins identified as differentially present in the medium fraction was the N-terminal extracellular domain of Ptk7. Conditioned media (CM) from both doxorubicin- and irradiation-induced senescent cells contained higher levels of soluble/shed form of Ptk7 (sPtk7) compared to CM from quiescent cells. The study identifies sPtk7 as a SASP factor that is released from senescent cells by metalloproteases and disrupts intestinal organoid morphology.
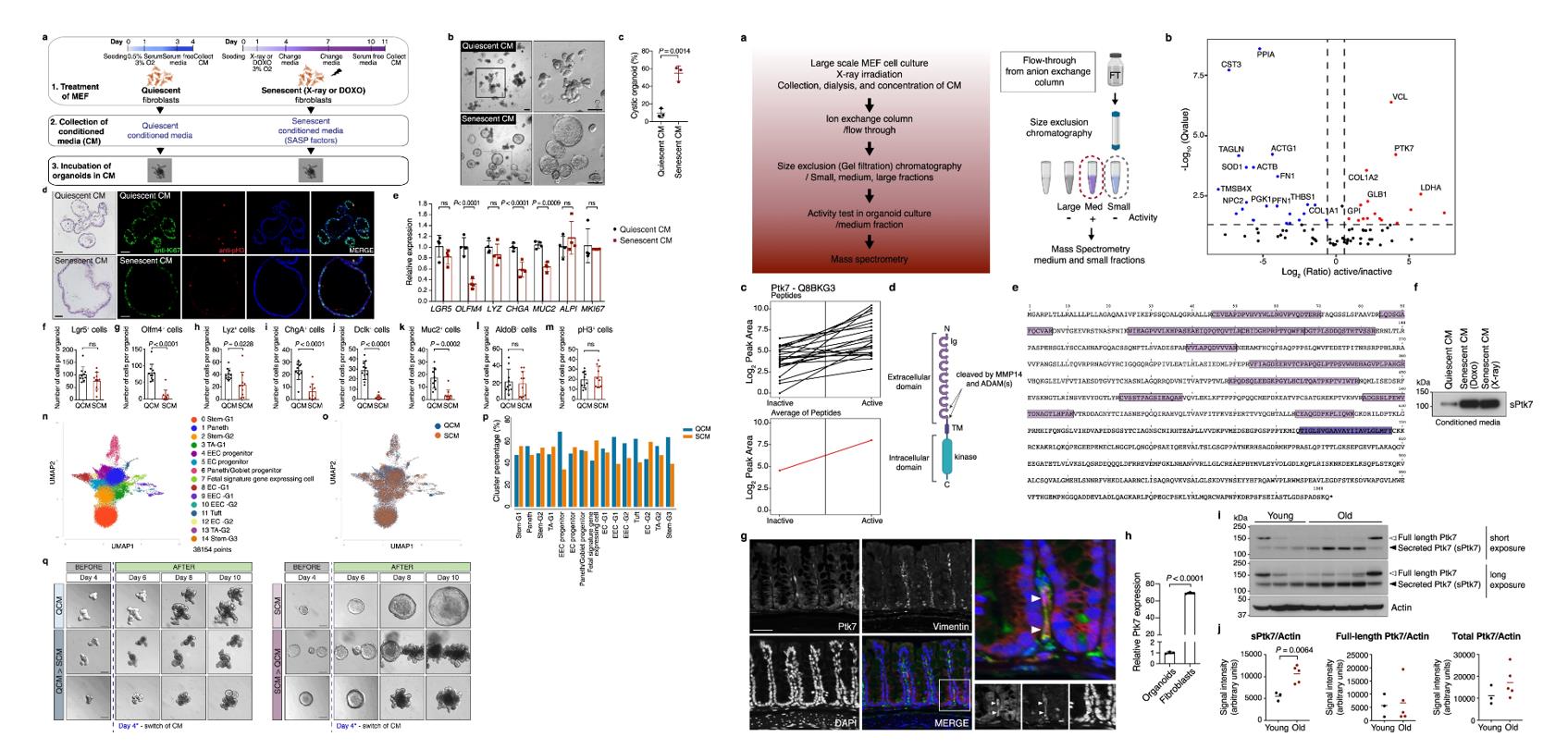 Fig. 1 Left: Conditioned medium from senescent fibroblasts causes cystic morphology of mouse intestinal organoids; Right: Identification of Ptk7 as a factor that causes cystic organoid phenotype.
Fig. 1 Left: Conditioned medium from senescent fibroblasts causes cystic morphology of mouse intestinal organoids; Right: Identification of Ptk7 as a factor that causes cystic organoid phenotype.
- Combined inhibition of FZD5, 7 and 8, as well as selective inhibition of FZD7 or FZD8, but not of FZD5, strongly suppressed the cystic phenotype induced by SCM. While both Wnt3a and Wnt5a dose-dependently increased organoids with cystic morphology, there was a noticeable difference in organoid size. The percentage of cystic organoids caused by Wnt5a was reduced by Ptk7 inhibition, sPtk7 and FZD7 bind both Wnt3a and Wnt5a, but sPtk7 bound Wnt5a with higher affinity, whereas FZD7 showed the opposite, these results suggest that Ptk7/FZD7/Wnt form a ternary complex to activate Wnt signaling.
- SCM increased the frequency and magnitude of cytosolic Ca2+ oscillations. Addition of SCM in the presence of anti-Ptk7 failed to induce any changes in Ca2+ oscillations. Ptk7 inhibition in unchallenged organoids decreased cytosolic Ca2+ oscillations, suggesting a role for endogenous Ptk7 in modulating Ca2+ signaling. Cytosolic Ca2+ release and subsequent activation of Calmodulin / CaMK signaling lead to the cystic organoid morphology.
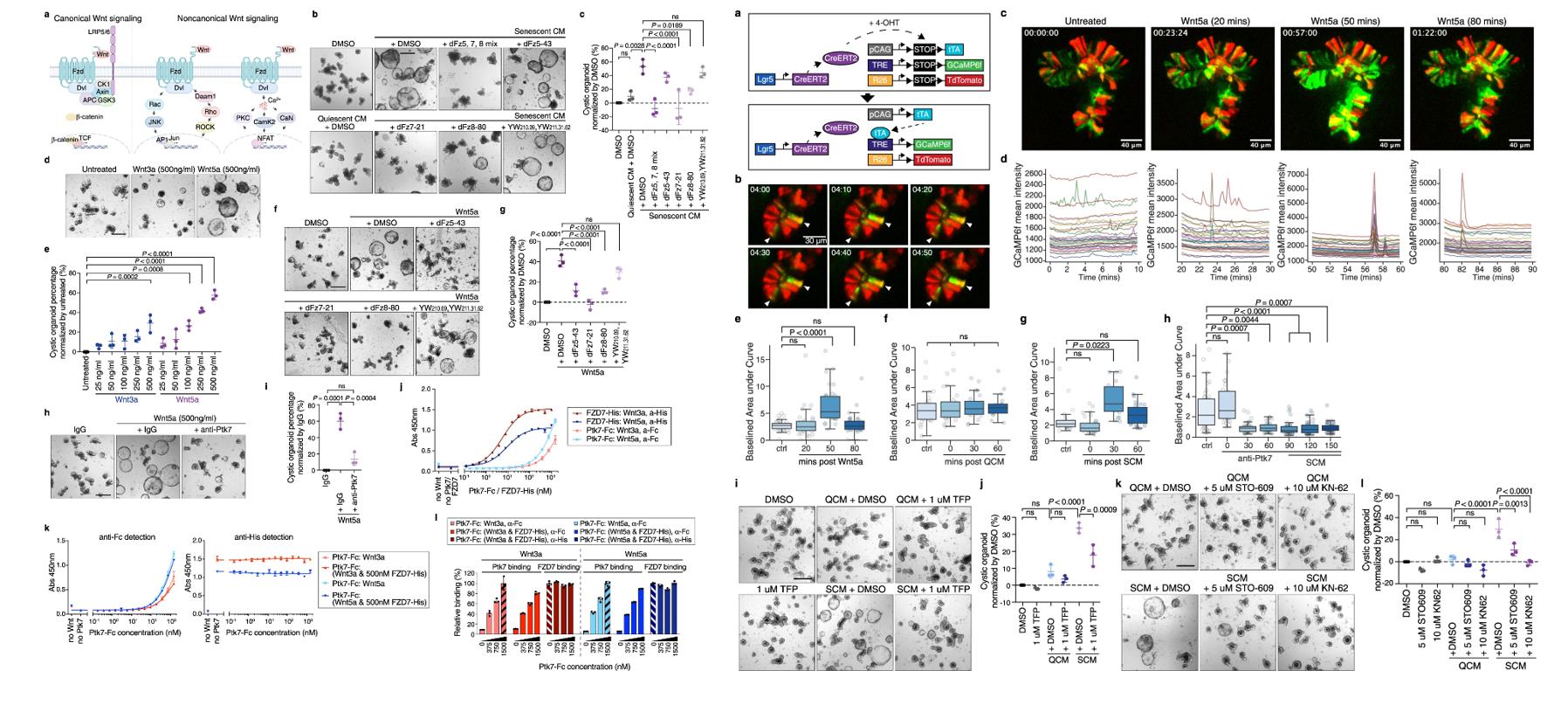 Fig. 2 Left: Ptk7 acts through noncanonical Wnt signaling; Right: Ptk7 modulates cytosolic Ca2+ oscillations in intestinal organoids.
Fig. 2 Left: Ptk7 acts through noncanonical Wnt signaling; Right: Ptk7 modulates cytosolic Ca2+ oscillations in intestinal organoids.
- Ptk7-dependent DEGs displayed strong enrichment for TEAD binding motifs, whereas Ptk7 independent DEGs showed enrichment for Hdac2, Hnf4g and Hnf1a binding motifs. Most Ptk7-dependent DEGs with TEAD binding motifs increased expression in response to SCM or Wnt5a, and are known YAP target genes. Altogether, these data suggest that the cystic organoid phenotype caused by Ptk7 or Wnt5a may result from elevated YAP/TEAD activity.
- Inhibition of nuclear YAP can rescue the cystic organoid phenotype. Ca2+ signaling has been implicated in the regulation of YAP, and inhibition of Ca2+ signaling components reversed SCM- or Wnt5a-induced nuclear translocation of YAP, as well as the cystic organoid phenotype.
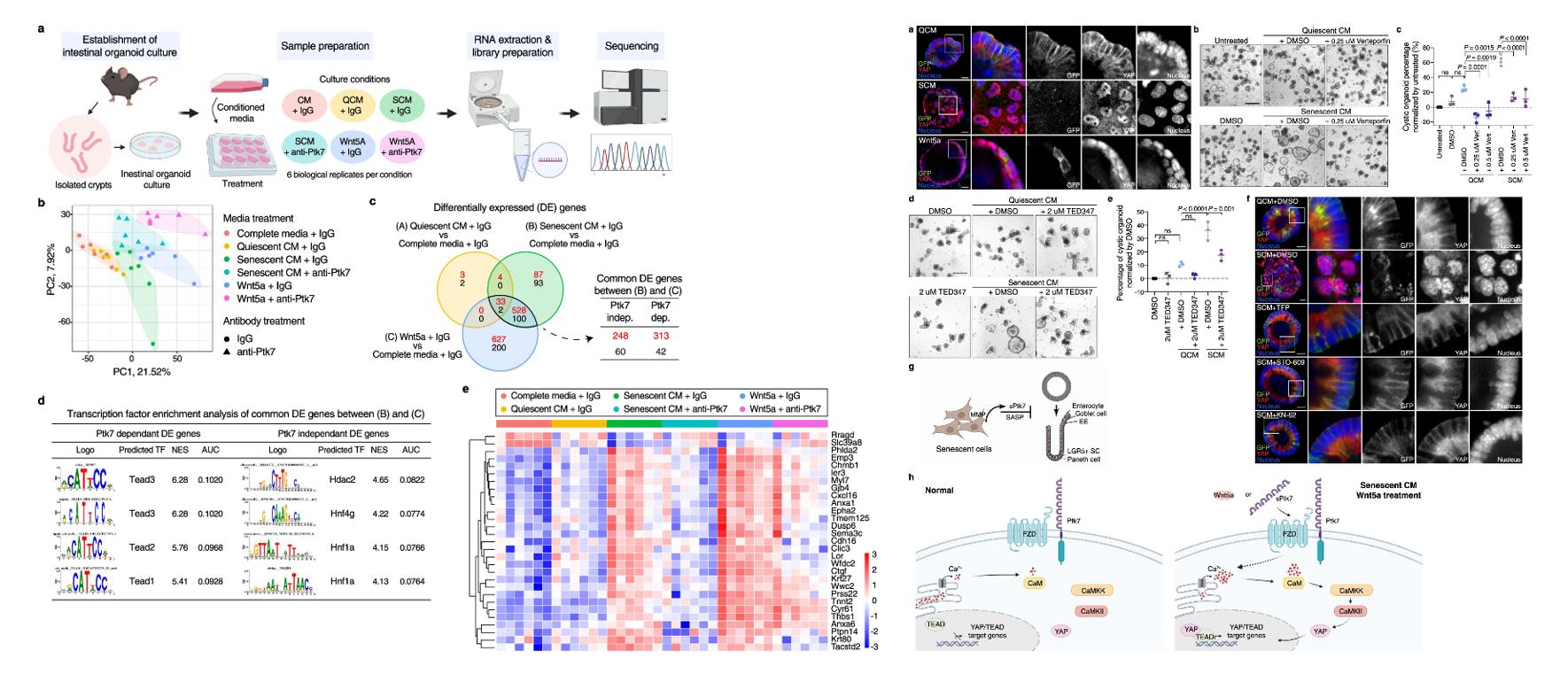 Fig. 3 Left: Transcriptomes of organoids shows enrichment of TEAD binding motifs among Ptk7-dependent DE genes; Right: Correlation between nuclear YAP localization and cystic organoid morphology and connection to Ca2+ signaling.
Fig. 3 Left: Transcriptomes of organoids shows enrichment of TEAD binding motifs among Ptk7-dependent DE genes; Right: Correlation between nuclear YAP localization and cystic organoid morphology and connection to Ca2+ signaling.
SUMMARY
- Here, using intestinal organoids as a model of tissue regeneration, we show that SASP factors released by senescent fibroblasts deregulate stem cell activity and differentiation and ultimately impair crypt formation.
- We identify the secreted N-terminal domain of Ptk7 as a key component of the SASP that activates non-canonical Wnt / Ca2+ signaling through FZD7 in intestinal stem cells (ISCs). Changes in cytosolic [Ca2+] elicited by Ptk7 promote nuclear translocation of YAP and induce expression of YAP / TEAD target genes, impairing symmetry breaking and stem cell differentiation.
RELATED PRODUCTS & SERVICES
Reference
- Yun J, et al. (2023). "Senescent cells perturb intestinal stem cell differentiation through Ptk7 induced noncanonical Wnt and YAP signaling." Nat Commun. 14 (1), 156.