Myeloid Cells Promote Deterioration of the Hematopoietic System
Nature Communications. 2022 Dec 10; 13(1): 7657
Authors: Feyen J, Ping Z, Chen L, van Dijk C, van Tienhoven TVD, van Strien PMH, Hoogenboezem RM, Wevers MJW, Sanders MA, Touw IP, Raaijmakers MHGP.
INTRODUCTION
- Innate and adaptive immune cells participate in the homeostatic regulation of hematopoietic stem cells (HSCs). HSC pools sustain multi-lineage hematopoiesis throughout the mammalian lifetime. They can do so by maintaining relative quiescence, self-renewal, and infrequent divisions during steady-state hematopoiesis. These critical processes are governed by ancillary cells in so-called stem cell niches, which include endothelial and mesenchymal cells in the mammalian hematopoietic system.
- Long-term experimental depletion of neutrophils (neutropenia) is not only of conceptual relevance to define their contribution to HSC biology but also of significant clinical relevance, as chronic neutropenia is a common hematological condition associated with leukemic transformation in congenital neutropenia syndromes.
METHODS
- To interrogate HSC responses to neutropenia, we exploited our previously reported mouse model of neutropenia, through targeted downregulation of Sbds in hematopoietic progenitor cells (HPCs) expressing the myeloid transcription factor CCAAT/enhancer binding protein α (C/EBPα, encoded by the Cebpa gene). Transplantation of embryonic day (E) 14.5 fetal liver cells isolated from Cebpacre/+ R26EYFP/+ SbdsF/F or Cebpacre/+ R26EYFP/+ Sbds+/+ embryos (CD45.2) into wild-type CD45.1 recipients enables the tracing of Cebpa Sbds-depleted or Sbds-proficient cells and their progeny based on enhanced yellow fluorescent protein (EYFP) expression.
- To explore the underlying molecular mechanisms governing long-term HSC quiescence and functional conservation of the hematopoietic system in neutropenia, we compared genes and transcriptional programs differentially expressed in HSCs in neutropenic mice vs. non-neutropenic controls to those reported as differentially expressed in young vs. old HSCs in literature.
- Mapping of IFN gene expression in other marrow cells, including niche subsets, T-cells, macrophages, eosinophils and NK cells using qPCR.
RESULTS
- Recipients of the Cebpacre/+ R26EYFP/+ SbdsF/F fetal liver cells (hereafter SbdsF/F or mutants) developed profound neutropenia with an absolute neutrophil count (ANC) in recipients of the Cebpacre/+ R26EYFP/+ Sbds+/+ fetal liver cells (hereafter Sbds+/+ or controls), which was stably sustained until sacrificing the animals at 4 months post-transplant. The HSCs in this model are Sbds proficient as indicated by the lack of EYFP+ cells within the HSC population and normal levels of Sbds transcripts as compared to reduced levels of expression as a known consequence of Sbds recombination in EYFP+ HPC1 cells.
- The number of dormant HSCs (LKS-Slam, CD34−), however, was maintained in neutropenia. HSCs in neutropenia were predominantly quiescent (in the G0 phase of the cell cycle), comparable to their control counterparts. In contrast, multipotent progenitors (MPPs) displayed significantly increased cycling. Contributions of the HSC compartment to this actively cycling progenitor pool were suggested by the reduced numbers of committed CD34+ HSCs.
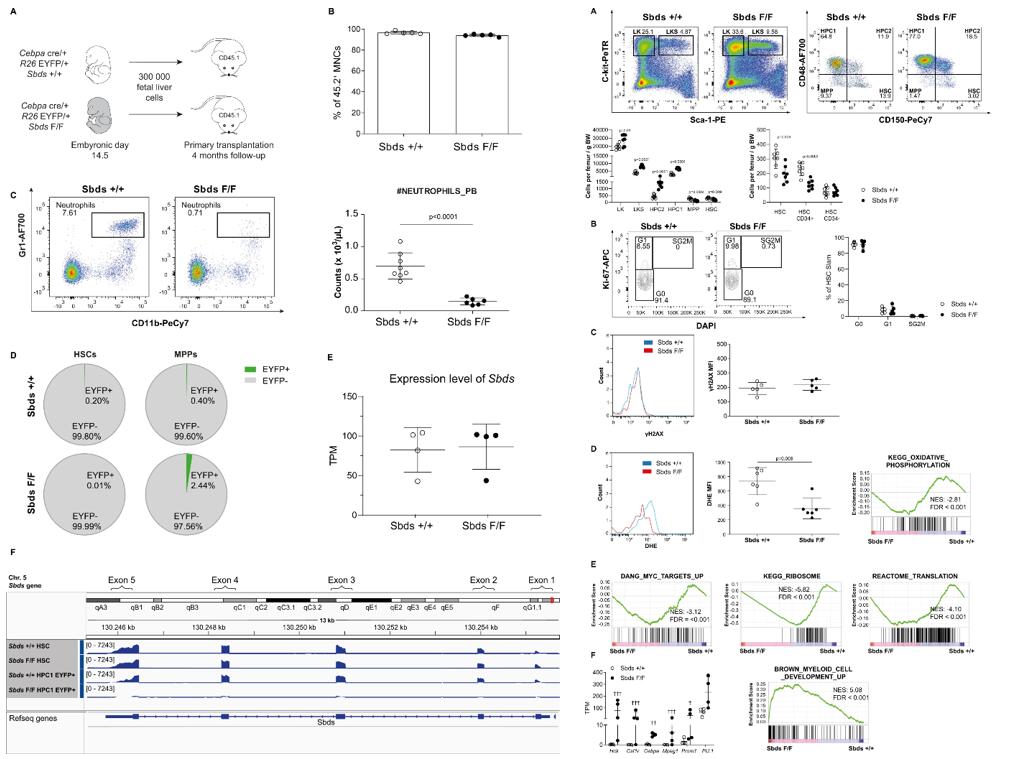 Fig. 1 Left: A mouse model of profound and sustained neutropenia with genetically intact HSCs; Right: The HSC population in neutropenia displays myeloid priming while remaining quiescent.
Fig. 1 Left: A mouse model of profound and sustained neutropenia with genetically intact HSCs; Right: The HSC population in neutropenia displays myeloid priming while remaining quiescent.
- Activation of interferon (IFN) signaling to be common to all reported datasets and HSCs in non-neutropenic vs. neutropenic mice. Expression of Iigp1, the gene encoding interferon-inducible GTPase1, was significantly reduced in HSCs in neutropenia. Furthermore, support for the activation of IFN signaling is provided by the upregulation of Sca1 in HSCs from primary and secondary transplant non-neutropenic mice, a known consequence of induction by interferons.
- Ifnγ expression in (NK1.1+) NK cells exceeded the expression of Ifnα4 and Ifnβ transcripts (>30-fold) and the expression of this gene in any other examined bone marrow cell type by >100-fold, identifying NK cells as the likely dominant source of interferons, and in particular IFNγ, in the steady-state bone marrow. Co-culture of HSPCs with NK cells, not neutrophils or eosinophils, resulted in consistent and robust activation of IFN signaling.
- In particular, the differentiated CD11b+ subset of NK cells was reduced in neutropenia. Genetic targeting of Sbds in NK cells was excluded by demonstrating that the vast majority of NK cells did not express EYFP and EYFP- NK cells were Sbds proficient. Furthermore, the small EYFP+ subset of NK cells did not differentially express Ifng.
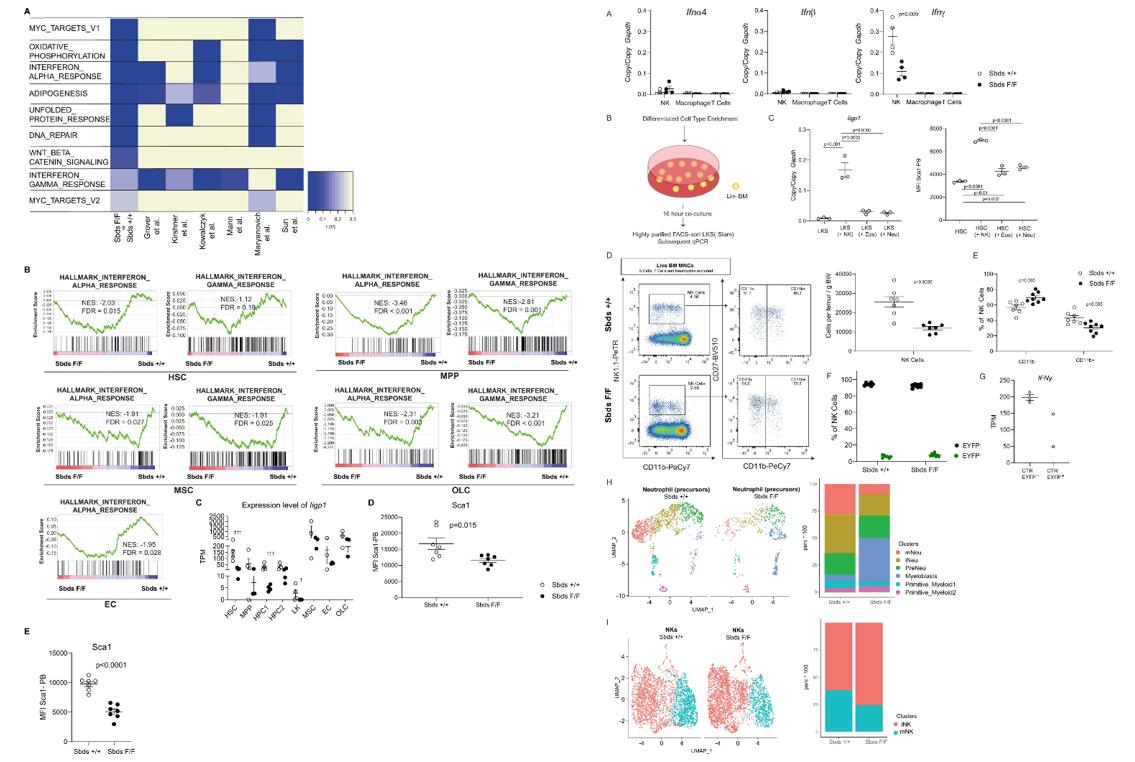 Fig. 2 Left: Interferon signaling is abrogated in hematopoietic and niche cells in neutropenia; Right: NK cells are the main source of interferons in the bone marrow, induce interferon signaling in HSPC, and are deficient in neutropenic mice.
Fig. 2 Left: Interferon signaling is abrogated in hematopoietic and niche cells in neutropenia; Right: NK cells are the main source of interferons in the bone marrow, induce interferon signaling in HSPC, and are deficient in neutropenic mice.
SUMMARY
- This study interrogates the contribution of myeloid cells, the most abundant cell type in the mammalian bone marrow, in a clinically relevant mouse model of neutropenia.
- Long-term genetic depletion of neutrophils and eosinophils results in activation of multipotent progenitors but preservation of HSCs. Depletion of myeloid cells abrogates HSC expansion, loss of serial repopulation and lymphoid reconstitution capacity and remodeling of HSC niches, features previously associated with hematopoietic aging.
- This is associated with mitigation of interferon signaling in both HSCs and their niches via reduction of NK cell number and activation. These data implicate myeloid cells in the functional decline of hematopoiesis, associated with activation of interferon signaling via a putative neutrophil-NK cell axis.
RELATED PRODUCTS & SERVICES
Reference
- Feyen J, et al. (2022). "Myeloid cells promote interferon signaling-associated deterioration of the hematopoietic system." Nat Commun. 13 (1), 7657.