Investigation of Simplified Physiologically-Based Pharmacokinetic Models
CPT Pharmacometrics and Systems Pharmacology. 2023 Mar; 12 (3): 333-345.
Authors: Yau E, Olivares-Morales A, Ogungbenro K, Aarons L, Gertz M.
INTRODUCTION
Physiologically-based pharmacokinetic (PBPK) modeling provides a powerful tool for integrating preclinical and in vitro data in a model for human pharmacokinetic (PK) predictions. It is often necessary to inform these models with animal or clinical data, update model parameters, and make the model more predictive for future applications. This work aimed to propose new mechanistic model structures with reduced complexity allowing for parameter optimization.
METHODS
- Two distinct approaches were investigated to simplify the PBPK model structure and/or reduce the number of parameters to facilitate parameter estimation, as shown in the following figure. Kinetic lumping reduces the complexity of the model from a mathematical point of view. In contrast, steady-state commonality in drug partitioning reduces the number of unknown parameters while mostly retaining the kinetic structure of the PBPK model.
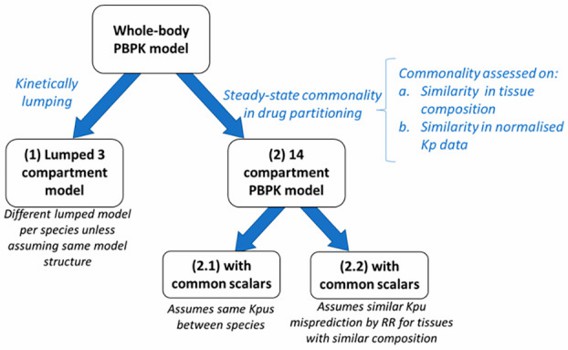 Fig. 1 Approaches investigated for simplifying a whole-PBPK model.
Fig. 1 Approaches investigated for simplifying a whole-PBPK model.
- Lumped body physiologically-based pharmacokinetic model with three compartments. For the three-compartment PBPK model, the lungs, venous, and arterial (serial tissues) compartments were lumped as one "central" compartment. This model specification implies a rapidly-(central), a moderately-(peripheral 1), and a slowly-(peripheral 2) equilibrating compartment. Tissues were attributed to one of the latter two compartments depending on the percentage difference and magnitude of the tissue time constants.
- Physiologically-based pharmacokinetic model with common Kpus or common scalars. Compared to the previously lumped PBPK model, the 14-compartmental PBPK model only makes the kinetic assumption that the lungs, venous, and arterial blood equilibrate quasi-instantaneously. This PBPK model is primarily based on similarity in tissue composition or steady-state drug partitioning while individual tissue blood flows and volumes are preserved compared to a full PBPK model.
- Browse our recommendations
Creative Bioarray provides professional PK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. In addition, our related services include PK/PD Biomarker Analysis, Bioavailability and Bioequivalence, Bioanalytical Package, Administration Routes and Biofluid Sampling, Blood-Brain-Barrier Assay, In Vivo Toxicity Study, etc.
RESULTS
- The proposed PBPK models were further investigated for parameter estimation using diazepam data in humans and rats. Initial exploration of structural models revealed that a linear two-compartment model best described the concentration-time profiles of diazepam in humans whereas a linear three-compartment model best described the rat data.
- All PBPK models could be successfully fitted to diazepam data in humans, and all estimated a Vss value within 20%-25% of the observed value (145 L). Estimated Kpu values from the investigated models were similar.
SUMMARY
In this work, two approaches for simplifying whole-body PBPK models were investigated. For diazepam, both approaches produced models which generally showed a good ability to describe in vivo data and to estimate parameters relating to drug clearance and distribution.
RELATED PRODUCTS & SERVICES
Reference
- Yau E, et al. (2023). "Investigation of simplified physiologically-based pharmacokinetic models in rat and human." CPT Pharmacometrics Syst Pharmacol. 12 (3), 333-345.