Investigation of Pharmacokinetic DDI Between Olaparib and Metformin
Cancer Chemotherapy and Pharmacology. 2024 Jan; 93 (1): 79-88.
Authors: Stanisławiak-Rudowicz J, Karbownik A, Szkutnik-Fiedler D, Otto F, Grabowski T, Wolc A, Grześkowiak E, Szałek E.
INTRODUCTION
Olaparib is a PARP (poly-ADP-ribose polymerase) inhibitor used for maintenance therapy in BRCA-mutated cancers. Metformin is a first-choice drug used in the treatment of type 2 diabetes. Both drugs are commonly co-administered to oncologic patients with add-on type 2 diabetes mellitus. Olaparib is metabolized by the CYP3A4 enzyme, which may be inhibited by metformin through the Pregnane X Receptor.
METHODS
- Male Wistar rats were assigned to three groups (eight animals in each group), which were orally administered: metformin and olaparib (IMET+OLA), vehiculum with metformin (IIMET), and vehiculum with olaparib (IIIOLA). Blood samples were collected after 24 hours.
- High-performance liquid chromatography (HPLC) with ultraviolet (UV) detection after a liquid-liquid extraction with a mixture of 1-butanol: n-heptane (50:50, v/v) was applied to measure the concentrations of metformin in the rats' plasma. Olaparib in the plasma samples was quantified with ultra-high-performance liquid chromatography.
- The following pharmacokinetic parameters of olaparib and metformin were calculated with the Pkanalix 2023R1 software (Lixoft, France): the elimination rate constant (ke), the absorption rate constant (ka), the half-life in the elimination phase (t1/2), the area under the concentration-time curve from zero to the last measurable concentration (AUC0-t), the area under the plasma concentration-time curve from zero to infinity (AUC0-∞), the apparent plasma drug clearance (Cl/F), and the apparent volume of distribution (Vd/F). The maximum plasma concentration (Cmax) and the time to reach the Cmax (tmax) were obtained directly from the measured values.
- Browse our recommendations
| Product/Service Types | Description |
| Drug-Drug Interaction | Creative Bioarray provides high-quality drug-drug interaction services, including identification of drug-metabolizing enzymes, CYP and UGT inhibition assays, and inhibition studies towards less common metabolizing enzymes, such as MAO, FMO, NAT, AOX, and CES. |
| CYP and UGT Reaction Phenotyping Assay | Creative Bioarray helps provide CYP and UGT reaction phenotyping assay for CYP1A2, CYP2B6, CYP2C8, CYP3A4, UGT1A1, UGT2B7, or other isoforms on clients' requests. |
| Pharmacokinetic and Toxicokinetic Studies | Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. |
RESULTS
Metformin did not affect the olaparib PK parameters. The AUC0-∞ IMET+OLA/IIIOLA ratio was 0.99. Olaparib significantly increased the metformin Cmax (by 177.8%), AUC0-t (by 159.8%), and AUC0-∞ (by 74.1%). The AUC0-∞ IMET+OLA/IIMET ratio was 1.74.
- When metformin was co-administered with olaparib, the AUC0-t and AUC0-∞ of metformin increased by 159.8% and 74.1%, respectively, as compared with the administration of metformin alone. In the presence of olaparib, the Cmax of metformin increased by 177.8%, whereas the Vd/F (65.1%) and Cl/F (43.4%) of metformin decreased. However, there were no significant differences between the two groups in the values of the other pharmacokinetic parameters. The values of the IMET+OLA/IIMET ratio for Cmax, AUC0-t, and AUC0-∞ were 2.78, 2.59, and 1.74, respectively.
- In comparison with the control group, the Vd/F (from 22.49 ± 24.81 to 12.51 ± 11.61 l) and Cl/F (from 7.84 ± 15.21 to 2.62 ± 2.07 l/h) of olaparib decreased when it was co-administered with metformin, but there was no statistical significance (p = 0.5995 and 0.8336, respectively). The values of the IMET+OLA/IIMET ratio for Cmax, AUC0-t, and AUC0-∞ were: 1.08, 0.99, and 1.02, respectively.
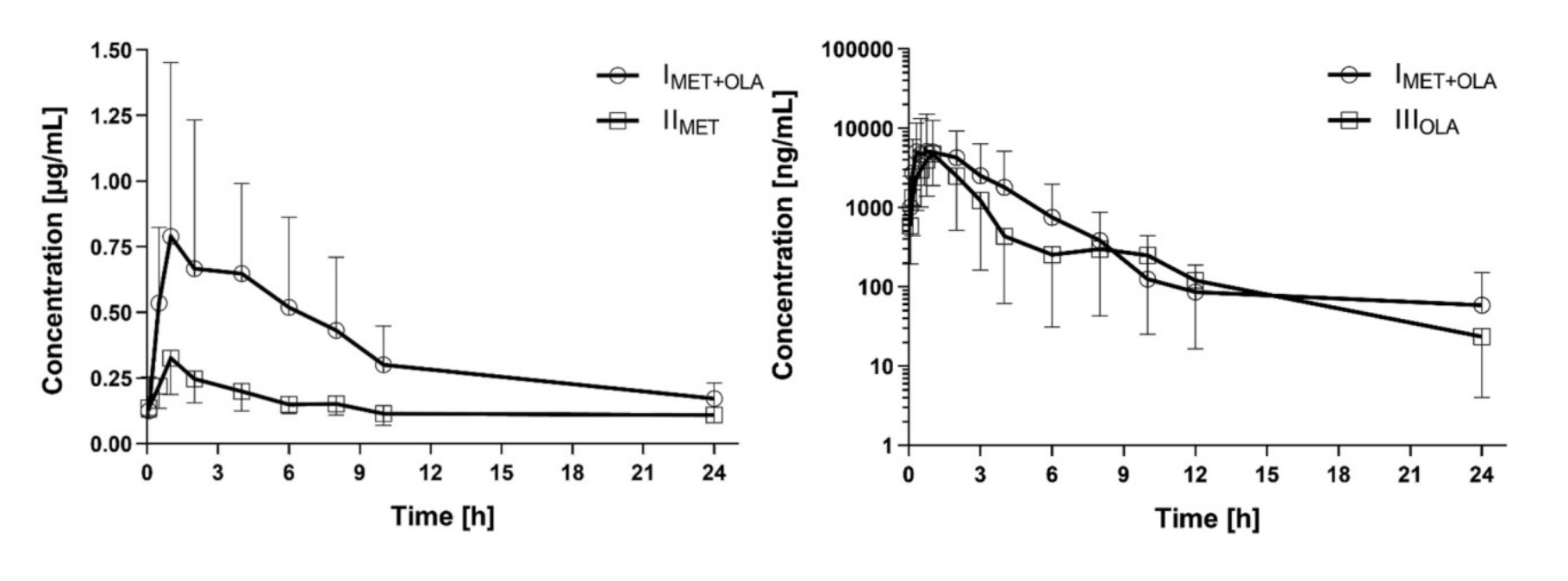 Fig. 1 Left: Metformin plasma concentration-time profiles (mean ± SD) in the rats that received metformin (IIMET) and metformin + olaparib (IMET+OLA); Right: Olaparib plasma concentration-time profiles (mean ± SD) in the rats which received olaparib (IIIOLA) and metformin + olaparib (IMET+OLA).
Fig. 1 Left: Metformin plasma concentration-time profiles (mean ± SD) in the rats that received metformin (IIMET) and metformin + olaparib (IMET+OLA); Right: Olaparib plasma concentration-time profiles (mean ± SD) in the rats which received olaparib (IIIOLA) and metformin + olaparib (IMET+OLA).
SUMMARY
A single dose of metformin did not affect the PK parameters of olaparib, nor did it inhibit the olaparib metabolism, but olaparib significantly changed the metformin pharmacokinetics, which may be of clinical importance.
RELATED PRODUCTS & SERVICES
Reference
- Stanisławiak-Rudowicz J, et al. (2024). "Bidirectional pharmacokinetic drug interactions between olaparib and metformin." Cancer Chemother Pharmacol. 93 (1): 79-88.