Impacts of NK Cell-Surface Receptors on CAR-NK Efficacy in Solid Tumors
Cell Death Discovery. 2024 Jan 20; 10 (1): 40.
Authors: Wang W, Liu Y, He Z, Li L, Liu S, Jiang M, Zhao B, Deng M, Wang W, Mi X, Sun Z, Ge X.
INTRODUCTION
Tumor immunotherapy is a treatment method to control and eradicate tumors by combating immune escape and reinstating the body's normal anti-tumor immune response. Chimeric antigen receptors (CARs) are fusion proteins, and the CAR structure of CAR-NK cells typically comprises three components: the extracellular antigen-binding region (usually scFv), the spacer and the transmembrane domain, and the intracellular activation domain.
Natural Killer (NK) cells, as unique innate immune cells, display rapid and potent cytotoxicity for cancer immunotherapy and pathogen clearance without prior sensitization or antigen recognition. CAR-NK cells are engineered to express CAR through genetic modification, connecting antibodies (or receptors) recognizing surface antigens of target cells (e.g. virus-infected cells and cancer cells) with signaling molecules required to activate immune cells. This modification can counteract inhibitory receptors, thereby enhancing NK cells' specific killing effect on target cells.
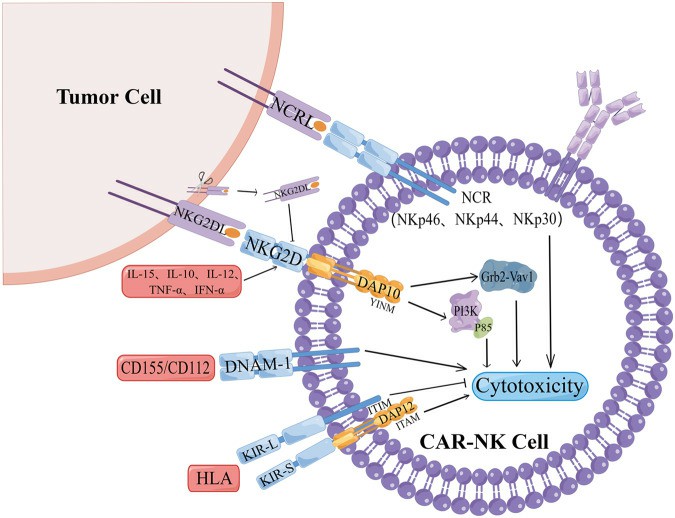 Fig. 1 The mechanism of NK cell surface receptors affecting the effect of CAR-NK.
Fig. 1 The mechanism of NK cell surface receptors affecting the effect of CAR-NK.
NKG2D/NKG2DLs
- NKG2D, encoded by the Klrk1 gene, serves as an activating receptor on the surface of NK cells. In humans, NKG2D forms a complex with the adapter protein DAP10, transmitting downstream signals through the charge in its transmembrane domain. NKG2D activates NK cells and delivers co-stimulatory signals to CD8 + T cells.
- The molecular structure of NKG2D allows it to bind many structurally distinct MHC I-like ligands (NKG2DLs). The expression of NKG2DLs is upregulated during malignant transformation, oxidative stress, and viral infection. MICA/B, mainly MICA, MICB, and ULBP1-6 in humans, is found in gastrointestinal epithelial tumors, as well as lung, breast, kidney, ovary, and prostate tumors.
- NKG2D is a multifunctional receptor that can directly bind to a variety of ligand molecule families expressed on the surface of target cells without antigen presentation, thereby activating or co-stimulating the immune effector. This activation is followed by the release of cytolytic proteins like perforin and granzymes, which mediate the killing of tumor cells. The NKG2D-mediated immune response plays a crucial role in tumor surveillance, and the NKG2D pathway can regulate tumor initiation and progression, which is essential for ensuring the efficacy of CAR-NK in solid tumors.
Killer Cell Immunoglobulin-Like Receptor (KIR)
- KIR is an Ig-like receptor molecule that can bind to certain HLA-I class molecules, serving as a key regulator of NK cell function. It is categorized into KIR2D (two domains) and KIR3D (three domains) based on the number of extracellular Ig-like domains. In KIR2D/3D, the cytoplasmic region containing the ITIM motif has a longer amino acid sequence, known as KLR2DL/3DL, acting as an NK cell inhibitory receptor.
- The transmembrane region contains a positively charged lysine, forming an NK cell-killing activated receptor when combined with negatively charged aspartic acid in the transmembrane region and the DAP-12 homodimer containing ITAM in the cytoplasmic region. NK cells undergo trogocytosis, a biological process involving the gnawing off of a part of the cell membrane and its surface molecules from antigen-presenting cells through the immune synapse. This process allows NK cells to transfer cell membrane surface substances from target cells to effector cells.
- CAR activation in NK cells facilitates the transfer of CAR cognate antigens from tumors to NK cells. However, this can lead to a decrease in tumor antigen density, weakening CAR-NK cell binding to targets. Additionally, it may induce self-recognition, sustained CAR-mediated binding, self-killing, and low reactivity in NK cells expressing granulocyte antigens (NKTROG+). This phenomenon can be counteracted by a dual-CAR system that combines an activating CAR directed against a cognate tumor antigen and an NK self-recognition inhibitory CAR that delivers a "don't kill me" signal to NK cells upon contact with TROG+. This system prevents granulocyte antigen-mediated cannibalism while preserving activating CAR signaling against tumor antigens and leads to enhanced CAR-NK cell activity.
DNAX Accessory Molecule 1 (DNAM-1)
Human DNAM-1 (CD226) is a type I transmembrane glycoprotein, approximately 65 kilodaltons (kDa) in size. DNAM-1 serves as an activating receptor that triggers NK cell-mediated cytotoxicity upon interaction with the ligands CD155 and CD112. Notably, the PVR/CD155 and Nectin-2/CD112 ligands of DNAM-1 are mainly expressed on solid tumor cells, especially those of epithelial and neuronal origin, and in normal tissue cells very little expression. Given that DNAM-1 ligands are expressed in many solid tumors, this treatment approach proves effective not only for neuroblastoma but also for colorectal cancer, breast cancer, ovarian cancer, lung cancer, pancreatic cancer, and other solid tumors exhibiting p53 dysfunction.
Natural Cytotoxicity Receptor (NCR)
NCR is a group of surface-activated receptors on NK cells, including NKp46 (NCR1, CD335), NKp44 (NCR2, CD336), and NKp30 (NCR3, CD337). All three are members of the immunoglobulin superfamily (IgSF), but have no homology with each other and typically play a killing role when KIRs lose their ability to recognize themselves. Studies have shown that NKp30 downregulation is associated with neuroblastoma metastasis and chemotherapy resistance. At present, there is a lack of data on the application of NCR in CAR-NK targeting solid tumors, which requires further verification.
Creative Bioarray Relevant Recommendations
At Creative Bioarray, our commitment to advancing immunotherapy and cellular therapy through the provision of high-quality, clinically relevant NK cell products has positioned us as a trusted partner for researchers, clinicians, and pharmaceutical developers alike.
| Cat. No. | Product Name |
| CSC-C4469X | Mobilized PB CD56+ NK Cells |
| CSC-C4486X | Frozen/Untouched Normal PB CD56+ NK Cells |
| CSC-C4491X | Frozen/Positively Selected Normal PB CD56+ NK Cells |
| CSC-C4523X | Cord Blood CD56+ NK Cells |
| CSC-C4724Z | C57BL/6 Mouse CD49b+ NK Cells (Pooled) |
| CSC-C4725Z | BALB/c Mouse CD49b+ NK Cells (Pooled) |
| CSC-C4728Z | Monkey CD16+ NK Cells |
| CSC-C5247S | Rabbit NK Cells |
| CSC-C5336S | Mouse NK Cells |
RELATED PRODUCTS & SERVICES
Reference
- Wang W, et al. (2024). "Breakthrough of solid tumor treatment: CAR-NK immunotherapy." Cell Death Discov. 10 (1): 40.