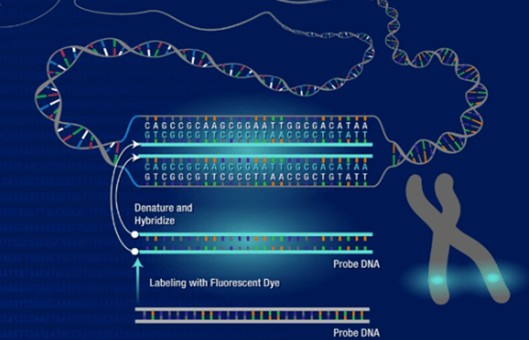
FFPE Tissue Preparation and FISH Protocol
GUIDELINE
Fluorescence in situ hybridization (FISH) in Formalin-fixed paraffin-embedded (FFPE) tissue samples is a widely used technique that allows for the detection and visualization of specific DNA or RNA sequences within the context of the tissue architecture. This approach is particularly valuable in the field of cancer diagnostics, as it enables the identification of chromosomal abnormalities and genetic alterations associated with various types of tumors.
METHODS
Slide preparation
- For FISH, 4-6 μm thick FFPE tissue sections should be used. Slides should be treated with an adhesive before mounting the tissue section. Throughout the entire procedure, unless otherwise indicated, the tissue section mustn't dehydrate.
Heat pretreatment
- Heat 50 ml tissue pretreatment solution (Reagent 1) in a porcelain wash jar or Coplin jar immersed in a water bath until it is either boiling or 98-100°C.
- Boil slides for 30 minutes.
- Wash in PBS or dH2O at room temperature (RT) for 2 x 3 minutes.
Enzyme digestion
- Cover tissue with 100-200 μl of enzyme reagent (Reagent 2) for 10 minutes at RT.
- Wash in PBS or dH2O at RT for 3 x 2 minutes.
- Dehydrate slides in a series of 70%, 85%, 95%, and 100% ethanol for 2 minutes each at room temperature, air dry, and proceed to denaturation and hybridization.
Pre-denaturation
- Remove the probe from the freezer and allow it to warm to room temperature (RT).
- Ensure that the probe solution is uniformly mixed with a pipette.
- Remove 10-15 μl (depending on the size of the tissue) of probe per test, and transfer it to a microcentrifuge tube. Quickly return the remaining probe to -20°C.
- Place the probe and the sample slide to prewarm on a 37°C (+/- 1°C) hotplate for 5 minutes.
- Spot 10-15 μl of probe mixture onto the sample and carefully apply a coverslip. Seal with rubber solution glue and allow the glue to dry completely.
Denaturation
- Denature the sample and probe simultaneously by heating the slide on a hotplate at 75°C (+/- 1°C) for 5 minutes.
Hybridization
- Place the slide in a humid, lightproof container overnight at 37°C (+/- 1°C).
Post-hybridization washes
- Remove the coverslip and all traces of glue carefully.
- Immerse the slide in 0.4xSSC (pH 7.0) at 72°C (+/- 1°C) for 2 minutes without agitation.
- Drain the slide and immerse it in 2xSSC, 0.05% Tween-20 at RT (pH 7.0) for 30 seconds without agitation.
- Drain the slide and apply 10-15 μl of DAPI antifade onto each sample.
- Cover with a coverslip, remove any bubbles, and allow the color to develop in the dark for 10 minutes.
Analyze
- View with a fluorescence microscope.
Creative Bioarray Relevant Recommendations
- Creative Bioarray provides a range of normal and diseased tissue blocks and tissue sections. Our high-quality tissues are banked under strict collection protocols to ensure that both paraffin-embedded and frozen tissues are all well-characterized, clinically annotated tissues needed for critical experiments.
- We also offer a range of different FISH services including metaphase and interphase FISH (chromosomal assignment and clone ordering), Fibre-FISH (Chromosome Painting), RNA-FISH (cell-based gene expression assay), M-FISH (multicolor karyotyping), 3D-FISH (on three-dimensionally preserved nuclei), Flow-FISH (quantify the length of telomeres), FISH on paraffin sections (analysis of archive material), ImmunoFISH (combined FISH and IHC).
NOTES
- Different incubation times may be required depending on tissue fixation. A 30-minute incubation is a recommended starting point.
- Depending on the tissue fixative used, different incubation times may be required. Excessive digestion will cause loss of nuclei and chromosome structure.