Fatty Acid Oxidation Assay
Fatty acid oxidation (FAO) is the main metabolic pathway in a variety of tissues and is particularly important during periods of glucose deficiency. In organs such as liver and skeletal muscle, FAO can provide more than 75% of cellular ATP, while in cardiac tissue it can be responsible for up to 90% of cellular energy requirements.
The primary pathway of fatty acids degradation is mitochondrial fatty acid β oxidation. Long chain fatty acids (LCFAs) enter the mitochondria in the form of acyl-carnitine. Once inside, acyl-CoA is released, undergoing an iterative four-step oxidation until the entire chain is oxidized to acetyl-CoA, while carnitine returns to facilitate further LCFA transport. The Acetyl CoA produced typically enters the TCA, although it can also fuel the production of ketone bodies in liver. Both TCA and β oxidation result in reduced equivalents (NADH and FADH2), which in turn drive ETC activity and subsequent ATP production.
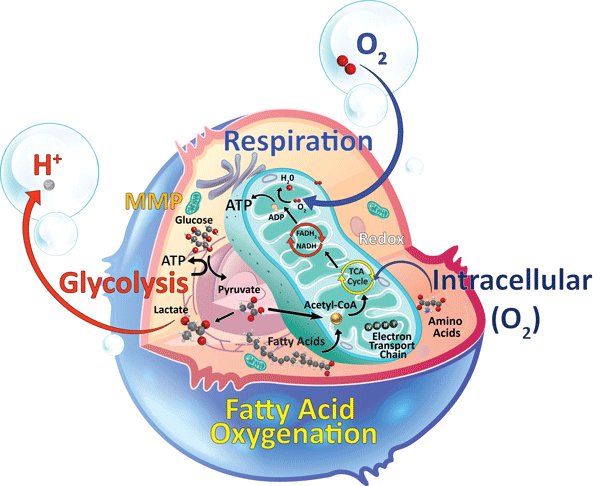 Figure 1. Fatty Acid Oxygenation.
Figure 1. Fatty Acid Oxygenation.
Materials
| FAO Conjugate (Oleate-BSA conjugate, 3mM) | FAO Control (BSA, 1.5mM) |
| L-Carnitine | FCCP |
| Etomoxir | Microplate reader |
| Sterile 96-well plate | Cell culture media |
| Base Measurement Media (dissolving FAO tablet in ddH2O, adjust pH to 7.4 using HCl and NaOH. Filter sterilize base media) | Base glucose deprivation media (glucose-free DMEM media, 1 mM glucose, 1 mM L-glutamine, 1% FBS, Penicillin/streptomycin solution (100 U/mL /0.1 mg/mL) |
| FA Measurement Media (FA-free Measurement Media + 150 µM FAO Conjugate) | FA-Free Measurement Media (Base Measurement Media + 0.5 mM L-Carnitine + 2.5 mM glucose) |
| Extracellular O2 Consumption Reagent (Prepare reagent as described in user manual) | Glucose (to prepare FA-Free Measurement Media) |
Assay Procedure
- Cell preparation
- Wash cells
- Add assay media to wells
- Treat cells
- Measurement
- Calculations
- Assay controls
1) Seed cells in a 96-well plate at a density of 5 x 104 cells/well in 200 µL culture medium.
2) Incubate overnight in a CO2 incubator at 37°C (typical incubation time > 14 hours).
3) Optional: Glucose deprivation step (Including a glucose deprivation step before performing the assay increases cellular dependence on FAO. For maximum FAO dependence, concentrations of L-Carnitine, glucose and FAO Conjugate should be optimized for each cell type).
4) Prepare Glucose deprivation media by adding 0.5 mM L-Carnitine to Base glucose deprivation media.
5) Wash cells twice with Base glucose deprivation media.
6) Add 200 µL of Glucose deprivation media.
7) Incubate overnight in a CO2 incubator at 37°C (typical incubation time > 14 hours).
1) Place the plate on a plate block heater set to assay temperature (typically 37°C) and remove spent culture media with an aspirator (be careful not to dislodge cells from the base of the wells).
2) With a multichannel or repeater pipette, add 100 µL of the pre-warmed FA-Free Media to each well. Repeat wash step one more time.
1) Signal control wells (wells with no cells) = 90 µL of pre-warmed FA Measurement Media.
2) Blank control wells = 90 µL of pre-warmed FA Measurement Media.
3) Sample wells = 90 µL of pre-warmed FA Measurement Media.
4) FA-Free control wells = 85 µL of FA-Free Measurement Media + 5 µL of BSA control.
Note: FA-Free Measurement Media is used as a control to measure O2 consumption without FAO Conjugate. BSA control is added to ensure that the free concentrations of test compounds are consistent between FA and FA-Free conditions. BSA concentration in FA-free control wells should be consistent with the BSA concentration in samples containing FAO Conjugate.
5) Add 10 μL of Extracellular O2 Consumption Reagent to each sample, FA-Free control and signal control wells. Do not add to blank control wells.
6) Add 10 µL of FA Measurement Media to blank control wells.
1) Add test compound or vehicle (typically 1-5 μL) to test wells (we recommend using 6-8 compound dilutions).
Note: We recommend keeping volume of added compound as low as possible to minimize any potential vehicle effects. Additional BSA control stock is added to wells without Oleate. Cells should be co-treated with FCCP if impact on maximal FAO is being determined. Etomoxir is used as a control. Ensure a minimum of 10 minutes between Etomoxir addition and assay measurement.
Seal each well with 100 µL of pre-warmed High Sensitivity Mineral Oil, taking care to avoid bubbles. Read plate immediately in a fluorescence microplate reader for extracellular oxygen consumption.
The data can be determined using slopes (m) calculated from the linear portion of each profile:
Exogenous FAO (Oleate supplied) = mOleate - mEtomoxir
Endogenous FAO (Oleate-free) = mOleate-free - mEtomoxir
Non-LC FAO (Etomoxir treated) = mEtomoxir – mSignal Control
1) FCCP exhibits a bell-shaped dose-response which can vary between cell types. The concentration which delivers maximum respiratory activity should be titrated for each cell type. Higher FCCP concentrations may be required when using FAO Conjugates as compared with glucose-based measurement due to the ability of BSA to bind FCCP.
2) FAO Conjugate is typically used at 150 μM. However, the concentration at which maximum respiratory activity is observed can be cell type dependent. Optimum concentration can be determined by measuring oxygen consumption at varying FAO Conjugate concentrations (typically 50-200 μM) in the presence of FCCP.
3) L-Carnitine is typically used at 0.5 mM. However, the optimum concentration to facilitate LCFA transport is cell type-dependent. Optimum concentration can be determined by measuring oxygen consumption at varying L-Carnitine concentrations in the presence of FCCP.
4) Etomoxir can exhibit ‘off-target’ effects if used at > 40 µM. Etomoxir efficacy can be reduced in presence of high serum and BSA concentrations. To maximize inhibition, Etomoxir can be pre-incubated in FA-Free Media prior to the addition of FAO Conjugate or BSA control.