Expansion and Differentiation of Human Hematopoietic Stem Cells
Cells. 2023 Mar 14; 12 (6): 896.
Authors: Bozhilov YK, Hsu I, Brown EJ, Wilkinson AC.
INTRODUCTION
The hematopoietic system plays an essential role in our health and survival. It is comprised of a range of mature blood and immune cell types, including oxygen-carrying erythrocytes, platelet-producing megakaryocytes, and infection-fighting myeloid and lymphoid cells. Self-renewing multipotent hematopoietic stem cells (HSCs) and a range of intermediate hematopoietic progenitor cell types differentiate into these mature cell types to continuously support hematopoietic system homeostasis throughout life.
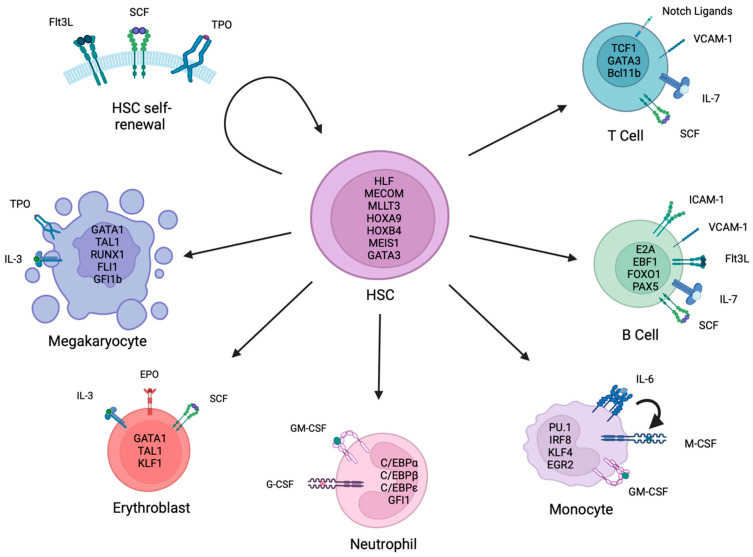 Fig. 1 Key factors and pathways involved in hematopoietic stem cell self-renewal and differentiation.
Fig. 1 Key factors and pathways involved in hematopoietic stem cell self-renewal and differentiation.
In Vitro Maintenance and Expansion of HSPCs
- A wide range of approaches have been tested in efforts to expand HSCs in vitro. HSC expansion protocols have powerful uses in basic research but also have direct translational applications to help boost donor HSC numbers for HSCT therapies. In vitro HSC culture conditions are also necessary for ex vivo HSC gene therapies.
- several signaling pathways play an important role in regulating HSC cell-fate decisions, including Wnt and Notch signaling. Other signaling pathways also play significant roles in HSC regulation, including the TGF-β/Smad, JAK/STAT, and PI3K/AKT pathways.
- Due to the limited success of in vitro HSC expansion using recombinant cytokines, the field has turned to implementing small-molecule approaches to improve in vitro HSC expansion, including tetraethylenepentamine (TEPA), stereogenic-1 (SR1), nicotinamide (NAM), prostaglandin E2 (PGE2), UM729, UM171, and others.
In Vitro Differentiation to Megakaryocytes
- Megakaryocytes are generated from HSCs through a stepwise process of differentiation. Several megakaryocyte differentiation protocols have been developed, which commonly employ the platelet markers glycoprotein IIb/IIIa (CD41) and glycoprotein Ib (CD42b), and/or measure cell ploidy to assess culture purity.
- Megakaryopoiesis is controlled by TFs which turn on the expression of megakaryocyte lineage-specific genes and suppress the transcriptional programs of other lineages. TFs found to be involved in this process include RUNX1, FLI1, GABPA, GATA2, LMO2, MYB, and NFE2.
In Vitro Differentiation to Erythrocytes
- In vitro production of mature erythrocytes from HSCs utilizes combinations of cytokines and/or stromal cells. It is commonly divided into several steps, which include erythroid lineage specification, erythroid progenitor expansion, and erythroid maturation.
- The purity of the cultures is commonly measured by the expression of the erythroid markers glycophorin A (CD235a), transferrin receptor (CD71), and/or Rhesus antigen (RhD), in combination with morphology analysis by May-Grünwald-Giemsa staining.
In Vitro Differentiation to Myeloid Cells
- The myeloid compartment includes granulocytes (neutrophils, eosinophils, and basophils), monocytes (macrophages and dendritic cells), and mast cells. As the most numerous and extensively studied cells, we focus here on neutrophils and monocytes.
- Numerous protocols have been developed to expand neutrophils ex vivo, often with the aim of eventual clinical translation. Differentiation towards the granulocyte lineage is typically achieved with a combination of SCF, FLT3L, IL-3, GM-CSF, and G-CSF.
- Several methods for the in vitro differentiation of monocytes have been developed and may facilitate a greater understanding of monocyte biology. These typically use a combination of Siglec-3 (CD33), FcγRI (CD64), the lipopeptide receptor CD14, and/or CD16 as monocyte markers to assess culture purity.
Creative Bioarray Relevant Recommendations
Creative Bioarray offers human hematopoietic stem cells and their adapted media, specifically Human Hematopoietic Stem Cells, Human Hematopoietic Stem Cell Growth Medium, Human Hematopoietic Expansion Medium, Human Hematopoietic Growth Medium, and others.
RELATED PRODUCTS & SERVICES
Reference
- Bozhilov YK, et al. (2023). "In Vitro Human Haematopoietic Stem Cell Expansion and Differentiation." Cells. 12 (6), 896.