Exosome-Mediated Delivery of Cas9 Ribonucleoprotein Complexes
Science Advance. 2022 Sep 16; 8(37): eabp9435
Authors: Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X.
INTRODUCTION
- CRISPR-Cas9 gene editing has emerged as a powerful therapeutic technology, but the lack of safe and efficient in vivo delivery systems. Delivery of Cas9 ribonucleoprotein (RNP) owns competitive advantages over other options; however, the large size of RNPs exceeds the loading capacity of currently available delivery vectors.
- Exosomes are naturally released nanovesicles by cells with the size ranging from 40 to 160 nm. Their inherent biocompatibility, transportation capability, bloodstream stability, and engineerability have made exosomes potential delivery vehicles for therapeutic purposes. Recently, endogenous exosomes obtained from the specific cells transfected with CRISPR-Cas9 plasmid have been successfully used to deliver Cas9 RNP to the target cells, indicating the promising potential of exosomes for RNP delivery.
METHODS
- To verify the successful isolation of exosomes from LX-2 cells, the researchers detected several exosome or cytoplasm markers by Western blotting, dynamic light scattering (DLS), transmission electron microscopy (TEM). In the end, we used electroporation methods to load Cas9 RNP into purified exosomes. To investigate the cellular uptake process, exosomes were labeled with red fluorescence CF640R wheat germ agglutinin (WGA).
- For the therapy of acute liver injury, we designed sgRNA-targeting PUMA and delivered exosomeRNP to investigate its therapeutic efficacy for the acute liver injury. we investigated the therapeutic potential of exosomeRNP in vivo. The targeting property of exosomeRNP was further proved by T7 endonuclease I (T7E1) assay.
- Genome editing of CcnE1 through exosomeRNP may also attenuate fibrosis initiation. We investigated the therapeutic potential of exosomeRNP in vivo. To induce parenchymal liver fibrosis, three intraperitoneal injections of CCl4 per week for 5 weeks were performed. For therapy, mice received exosomeRNP nanocomplexes 7 days after the first CCl4 injection and twice a week thereafter.
- We designed sgRNA-targeting KAT5 and developed exosomeRNP to treat HCC. One week after the hepatic portal vein injection of luciferase-transfected Huh-7 (Huh-7-luci) cells, mice were intravenously injected with different treatments (PBS, RNP, exosome + RNP, exosomeRNP, and exosomemock RNP), and the tumor growth was evaluated using in vivo bioluminescence imaging.
RESULTS
- The lysate from LX-2-derived exosomes expressed exosome-specific markers CD63 and TSG101. LX-2-derived exosomes, with the size ranging from 50 to 200 nm, showed typical saucer-shaped nanovesicles. Cas9 RNP has been successfully loaded into the purified exosomes through an electroporation-based method, and no obvious changes on the size and morphology of exosomes can be detected. exosomeRNP delivery achieved an obvious indel frequency at different loci, suggesting that the delivery of exosomeRNP resulted in efficient genome editing in these different loci.
- AST and ALT levels and PUMA protein expression were significantly decreased after the exosomeRNP treatment. Reduced centrilobular cell necrosis and hyperemia was clearly observed in the mice treated with exosomeRNP complexes. Furthermore, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL)-positive cells were markedly reduced of mice received with exosomeRNP treatment, in sharp contrast with other treatments.
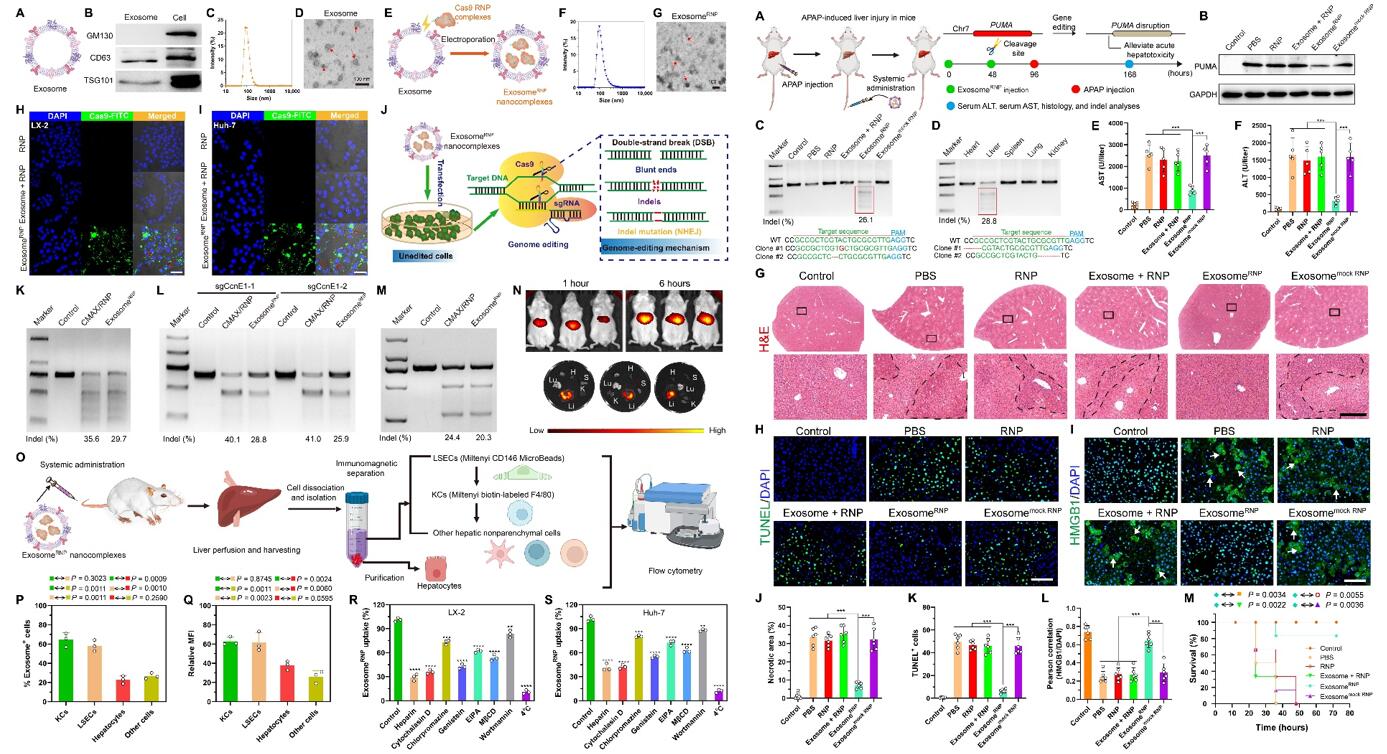 Fig. 1 Left: Characterization, genome-editing activity, biodistribution and cellular uptake mechanism of exosomeRNP; Right: ExosomeRNP-mediated genome editing suppressed APAP-induced acute liver injury and lethality.
Fig. 1 Left: Characterization, genome-editing activity, biodistribution and cellular uptake mechanism of exosomeRNP; Right: ExosomeRNP-mediated genome editing suppressed APAP-induced acute liver injury and lethality.
- The obvious digestion bands were only observed in the liver tissue among various organs, which indicated strong liver-targeting ability of exosomeRNP. ExosomeRNP treatment resulted in significant reduced CcnE1 and α-SMA protein expression. As evidenced by H&E staining, Ishak scoring, collagen1α1 expression, and hydroxyproline content determination, therapeutic intervention with exosomeRNP targeting CcnE1 largely attenuated fibrosis initiation.
- The mice treated with exosomeRNP-targeting KAT5 displayed the smallest tumor volume and the weakest intensity of bioluminescence. In addition, protein analysis indicated that the KAT5 expression level was remarkably reduced after the exosomeRNP treatment. As compared with other groups, the survival of the mice treated with exosomeRNP was significantly prolonged as well. And a similar tendency was observed by the histological staining. Collectively, these results demonstrated that exosomeRNP targeting KAT5 produced excellent anticancer activities in orthotopic HCC.
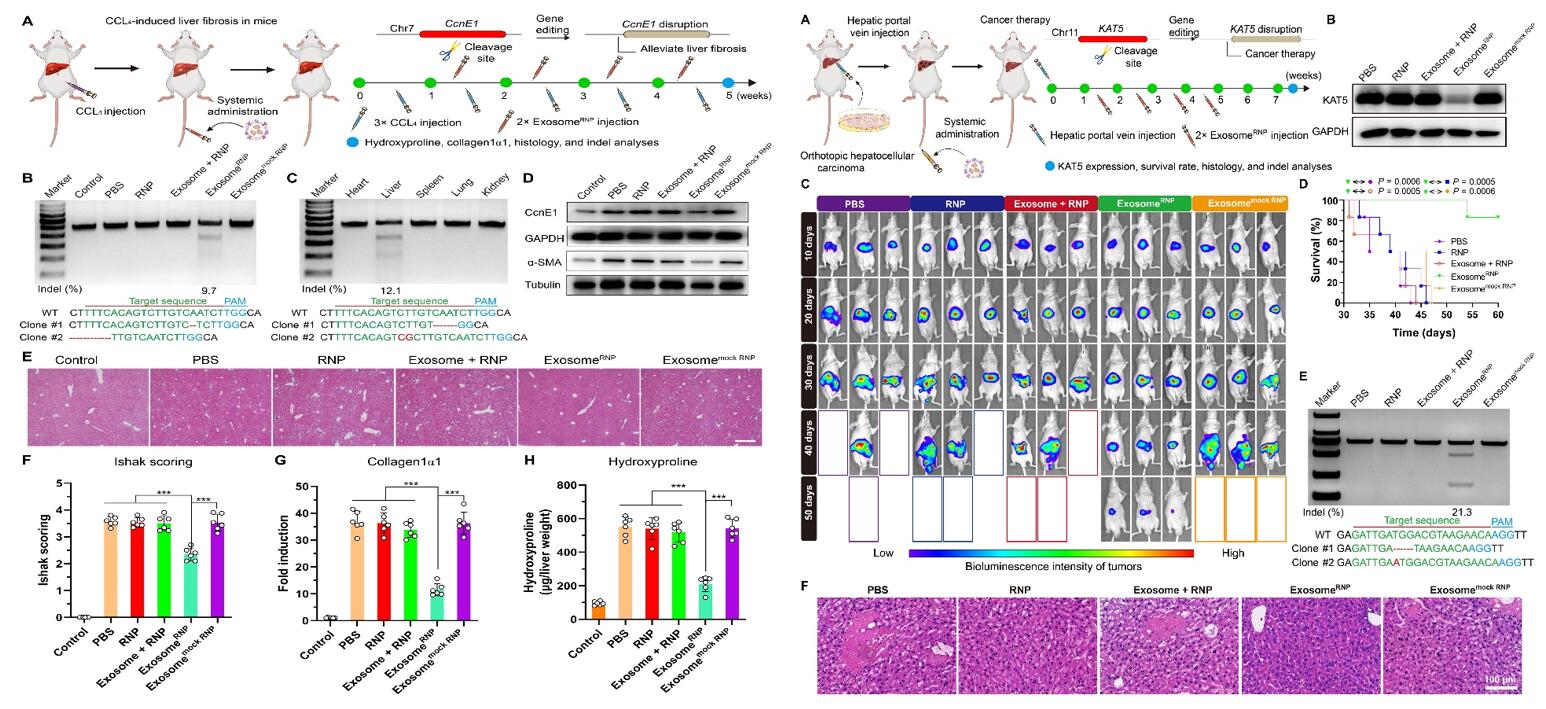 Fig. 2 Left: exosomeRNP-mediated genome editing suppressed CCL4-induced liver fibrosis; Right: exosomeRNP-mediated genome editing for orthotopic HCC therapy.
Fig. 2 Left: exosomeRNP-mediated genome editing suppressed CCL4-induced liver fibrosis; Right: exosomeRNP-mediated genome editing for orthotopic HCC therapy.
SUMMARY
- This study reports a previously unidentified genome editing delivery system, named exosomeRNP, in which Cas9 RNPs were loaded into purified exosomes isolated from hepatic stellate cells through electroporation.
- exosomeRNP facilitated effective cytosolic delivery of RNP in vitro while specifically accumulated in the liver tissue in vivo. exosomeRNP showed vigorous therapeutic potential in acute liver injury, chronic liver fibrosis, and hepatocellular carcinoma mouse models via targeting p53 up-regulated modulator of apoptosis (PUMA), cyclin E1 (CcnE1), and K (lysine) acetyltransferase 5 (KAT5), respectively.
- The developed exosomeRNP provides a feasible platform for precise and tissue-specific gene therapies of liver diseases.
RELATED PRODUCTS & SERVICES
Reference
- Wan T, et al. (2022). "Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases." Sci Adv. 8 (37), eabp9435.