Design of Human Stem Cell-Derived Islets
Cell Reports Medicine. 2023 Jan 17; 4 (1): 100879.
Authors: Gerace D, Zhou Q, Kenty JH, Veres A, Sintov E, Wang X, Boulanger KR, Li H, Melton DA.
INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disease that results in the destruction of the insulin-producing beta cells of the pancreas. To address the shortage of islet material, several protocols have been developed to steer the in vitro differentiation of human induced pluripotent stem cells (iPSCs) into functional stem cell-derived islets (SC-islets) that include glucose-response beta cells. More recently, genetic engineering of SC-islets to evade immune recognition has been attempted.
METHODS
- We targeted the expression of PD-L1 and the HLA-E long-chain fusion to the GAPDH locus of human embryonic SCs (hESCs) and used luminescence as a reporter of cell viability. We then transplanted WT, PD-L1, and B2P SC-islet cells under the kidney capsule of B6/albino mice to assess their ability to survive against Xeon-rejection. We used bioluminescence imaging to track SC-islet cell survival.
- We co-cultured IFN-γ-treated SC-islet cells with human PBMCs in vitro, then performed bulk RNA sequencing on IFN-γ-stimulated WT CD49a+ SC-β cells and assessed T cell ligand expression. We therefore re-analyzed single-cell RNA sequencing (scRNA-seq) expression datasets from our existing in vitro SC-β cell differentiation, an in vivo SC-β cell transplantation, and primary human islets, focusing on the expression of NK cell ligands.
- To identify potential ligand-receptor pathways that may result in the survival of B2M−/− SC-islet cells when co-cultured with IL-2 pre-activated NK cells, we assessed the surface expression of NK cell activating and inhibitory receptors on freshly isolated and IL-2 pre-activated NK cells. We assessed the ability of HLA-deficient SC-islet cells to normalize hyperglycemia in diabetic, NSG double knockout (DKO) mice prior to PBMC injection. Once blood glucose levels had been normalized, we injected HLA-A2− (mis-matched) PBMCs and assessed allo-rejection by monitoring blood glucose levels.
RESULTS
- GAPDH-targeting plasmids were used to create five hESC lines, wild type (WT), HLA-deficient (B2M−/−), PD-L1-expressing (PD-L1), HLA-deficient/PD-L1-expressing (B2P), and HLA-deficient/HLA-E-expressing (BEC). All five gene-modified hESC lines successfully differentiated into Nkx6.1+/C-peptide+ SC-β cells. PD-L1 over-expression is not sufficient to protect HUES8-derived SC-islet cells from Xeon-rejection and that the additional ablation of HLA expression does not improve xenograft survival in our in vivo model.
- Since Xeon-rejection does not mimic allograft (allo) rejection, we next chose to assess the survival of our HLA engineered SC-islet cells in an allogeneic setting. In the co-culture assays, B2M−/− and BEC SC-islet cells possess significantly improved survival compared with WT, and there was no significant difference in the survival of all gene-modified SC-islet cells cultured with CD3/CD28 activated cells.
- IFN-γ stimulation upregulated expression of the T cell co-inhibitory ligands PD-L1 and LGALS9 (galectin 9) in SC-β cells, members of the tumor necrosis factor (TNF) receptor superfamily HVEM and CD40 and the major histocompatibility complex (MHC)-associated gene butyrophilin subfamily 3 members A1 (BNT3A1). These results suggest that in the absence of classical HLA-TCR signaling due to HLA knockout, other co-stimulatory and co-inhibitory T cell ligands are expressed in SC-β cells that may influence T cell function.
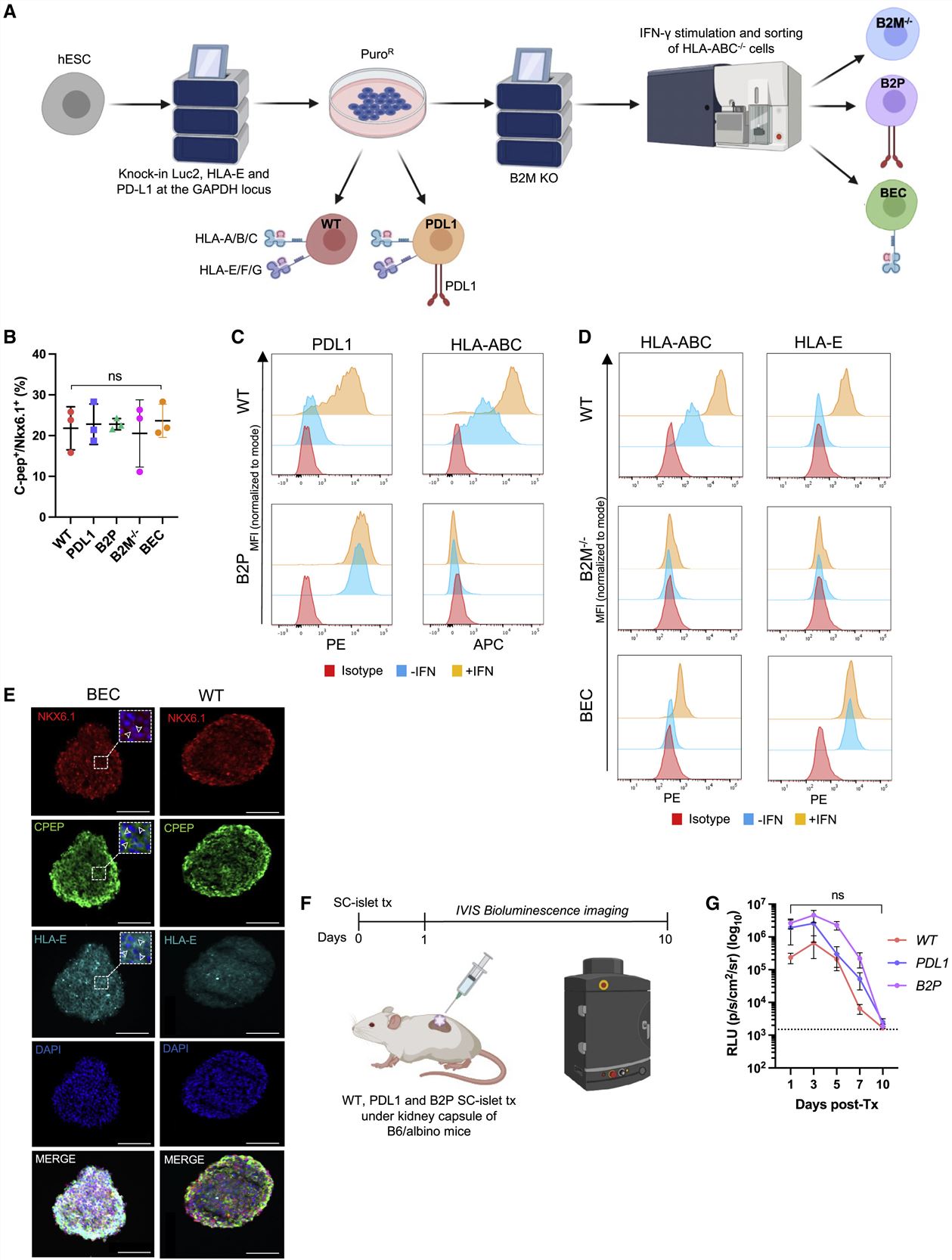
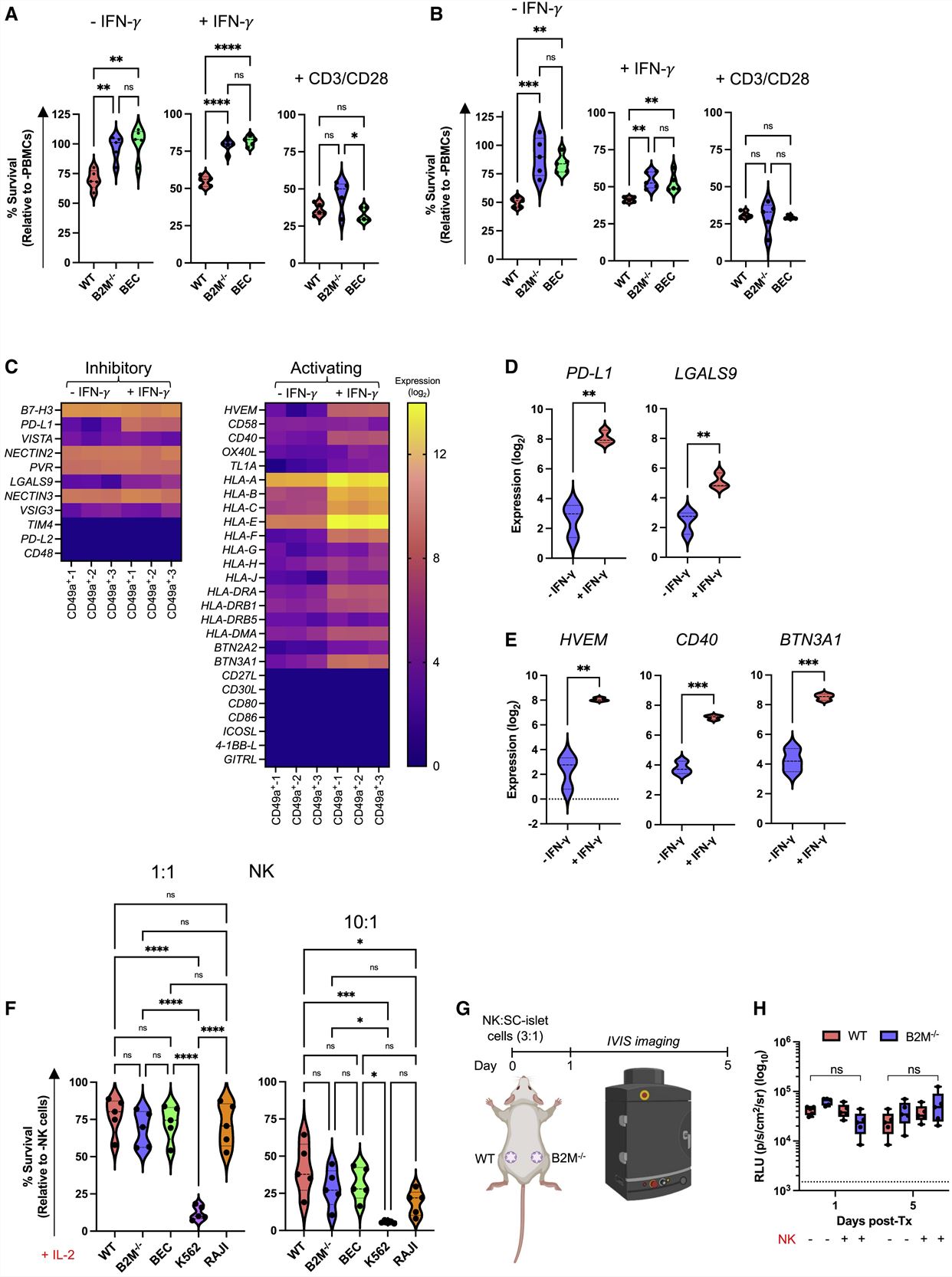
Fig. 1 Left: Generation of immune-evasive SC-islet cells; Right: In vitro co-culture of immune-evasive SC-islet cells with allogeneic human immune cells.
- scRNA-seq and flow cytometry suggests that SC-β cells possess a diminished NK cell activating ligand phenotype. The expression of the activating receptors NKp30, NKp46, DNAM-1, and NKG2D were upregulated upon IL-2 pre-activation in both CD56dim and CD56high NK cells, whereas NKp80 was downregulated. When B2M−/− SC-islet cells were co-cultured with pre-activated NK cells in the presence of anti-SIRPα and anti-TIGIT neutralizing antibodies, SC-islet cell survival decreased.
- By 10 weeks post-transplantation, there was no significant difference in blood glucose levels of mice transplanted with WT and B2M−/− SC-islet cells. At 7 weeks post-PBMC injection, in vivo glucose-stimulated insulin secretion (GSIS) assay showed that animals transplanted with WT SC-islet cells lost in vivo graft function, while some graft function was observed in animals transplanted with B2M−/− SC-islet cells.
- We engineered human pluripotent stem cells to express modified IL-2, IL-10, and TGF-β from the GAPDH locus. When WT and 2B10 SC-islet cells were co-cultured with human PBMCs in vitro, 2B10 SC-islet cells showed significantly improved survival compared with their WT counterparts. When 2B10 SC-islet cells were transplanted in B6/albino mice, they survived up to 9 weeks post-transplantation, and 2B10 grafts contained surviving insulin-producing cells and Tregs localized within the graft.
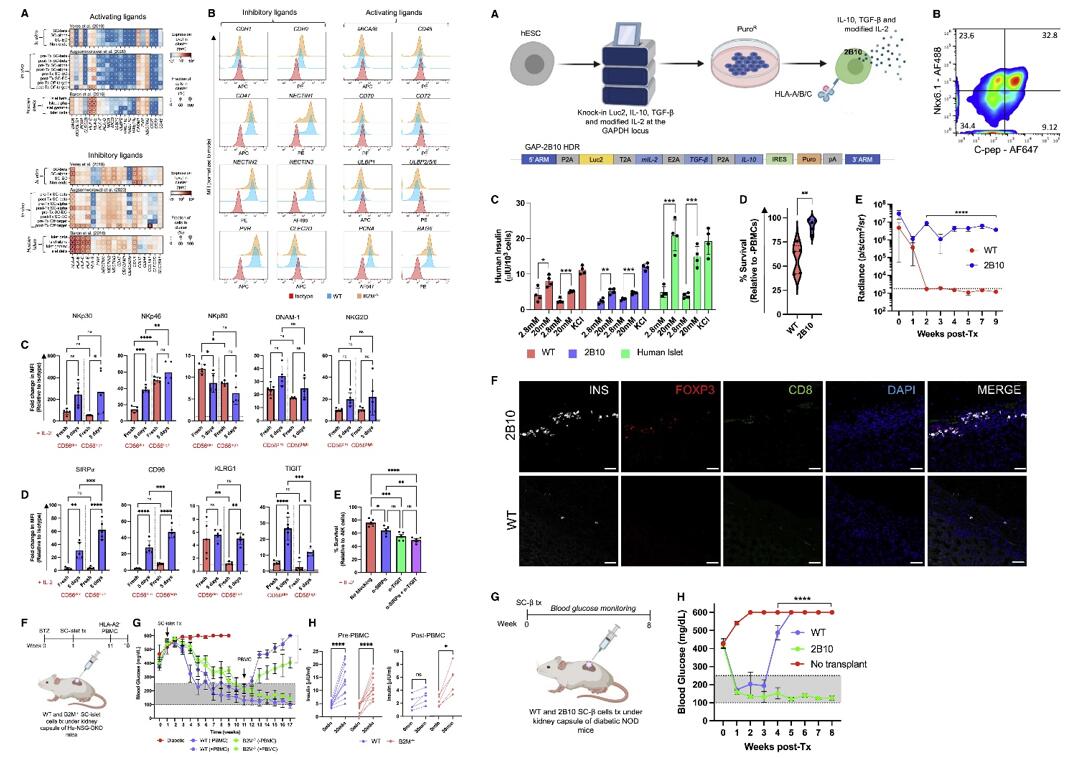 Fig. 2 Left: SC-islet cell NK cell ligand profile; Right: Immune-tolerizing SC-islet cells survive xeno-rejection.
Fig. 2 Left: SC-islet cell NK cell ligand profile; Right: Immune-tolerizing SC-islet cells survive xeno-rejection.
SUMMARY
- Targeting human leukocyte antigens (HLAs) and PD-L1 alone does not sufficiently protect SC-islet cells from xenograft (Xeon)- or allograft (allo)-rejection. As an addition to these approaches, we genetically engineer SC-islet cells to secrete the cytokines interleukin-10 (IL-10), transforming growth factor β (TGF-β), and modified IL-2 such that they promote a tolerogenic local microenvironment by recruiting regulatory T cells (Tregs) to the islet grafts.
- Genetically engineering human embryonic SCs (hESCs) to induce a tolerogenic local microenvironment represents a promising approach to provide SC-islet cells as a cell replacement therapy for diabetes without the requirement for encapsulation or immunosuppression.
RELATED PRODUCTS & SERVICES
Reference
- Gerace D, et al. (2023). "Engineering human stem cell-derived islets to evade immune rejection and promote localized immune tolerance." Cell Rep Med. 4 (1), 100879.