FISH Protocol For Paraffin Embedded Tissue Samples
Paraffin-embedded tissue is one of the most widely practiced methods for sample preservation and archiving. It is estimated that, over a billion tissue samples, most of them paraffin-embedded, are being stored in numerous hospitals, tissue banks, and research laboratories in the word. These archived samples could potentially provide a wealth of information in retrospective molecular studies, especilally FISH.
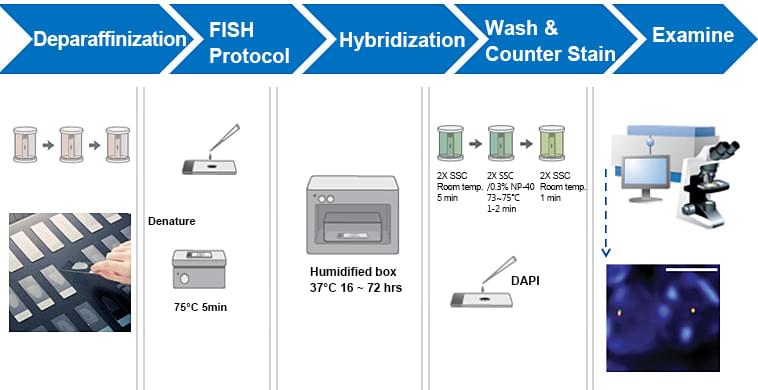
Deparaffinization of paraffin-embedded tissue sections
| 1) | Paraffin-embedded tissue must be sliced at a thickness of 4-10 μm using a microtome and mounted onto a microscope slide |
| 2) | Prewarm 40 ml 0.01N HCL in a jar in 37°C water bath |
| 3) | Age slide at 90°C for 25 min |
| 4) | Immerse the slide-mounted tissue section in 100% xylene for 15 minutes; repeat in fresh 100% xylene for an additional 15 minutes |
| 5) | Immerse the slide in 100% ethanol for 5 minutes; repeat in fresh 100% ethanol for an additional 5 minutes |
| 6) | Air dry slide |
| 7) | Pre-warm a jar with 10 mM Citric acid to 80°C in water bath |
| 8) | Place slide into Pepsin solution for 30 min |
| 9) | Rinse slide in 70% ethanol 30 second |
| 10) | Air dry slide and check slide for proper digestion; reveal dark distinguishable cells |
| 11) | Dehydrate slide through 70%, 85% and 100% Ethanol each 2 min |
| 12) | Air dry slide and proceed to hybridization |
Co-denaturation and hybridization
Prepare probe mix per hybridization for 22 mm2 coverslip. Add the probe on the processed tissue slide and place coverslip taking care that there are no air bubbles.
Hybridize the slides with following program:
- Denaturation: 83°C for 3 min.
- Hybridization: 37°C over night
Creative Bioarray provides services for entire FISH process with unprecedented analytical accuracy, you can find more at: Histology Services