Porcine Bone Marrow Mesenchymal Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
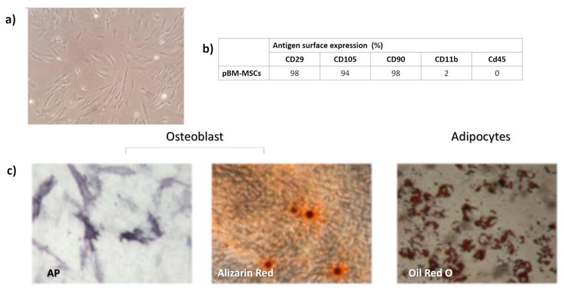
Porcine bone‑marrow mesenchymal stem cells (PB‑MSCs) are adult stromal cells that are isolated from the marrow cavity of the pig bone, most often from the iliac crest, tibia or femur. PB‑MSCs are phenotypically similar to their human counterparts and express the classic MSC markers CD105, CD73 and CD90 at >95 % positivity, and are negative for hematopoietic antigens like CD45, CD34 and HLA‑DR, fulfilling the minimal ISCT criteria for animal MSCs. Their doubling time averages 50-55 h and remains stable for >40 cumulative population doublings, especially when isolated by the rapid RBC‑lysis method, which yields the highest cell numbers and enables intra‑operative, one‑step procedures.
PB‑MSCs demonstrate the ability to differentiate into multiple lineages when cultured in vitro conditions. Osteogenic induction (β‑glycerophosphate, dexamethasone, ascorbic acid) results in alkaline‑phosphatase activity and Alizarin‑Red calcium deposits; adipogenic protocols (IBMX, insulin, indomethacin) result in Oil‑Red‑O‑positive lipid droplets; and chondrogenic micromass cultures develop into glycosaminoglycan‑rich matrix stained by Alcian‑Blue and collagen‑II immunoreactivity.
Functionally, PB‑MSCs secrete immunomodulatory cytokines (TGF‑β, IL‑10, PGE₂) and neurotrophic factors similar to human MSCs, and have been used in pre‑clinical studies of cartilage repair, bone regeneration, myocardial protection and transplant tolerance. They have been shown to have a comparable immunosuppressive capacity to human MSCs, making them a useful large‑animal model for translational cell‑therapy studies and their autologous availability and robust expansion may simplify manufacturing for future clinical applications.
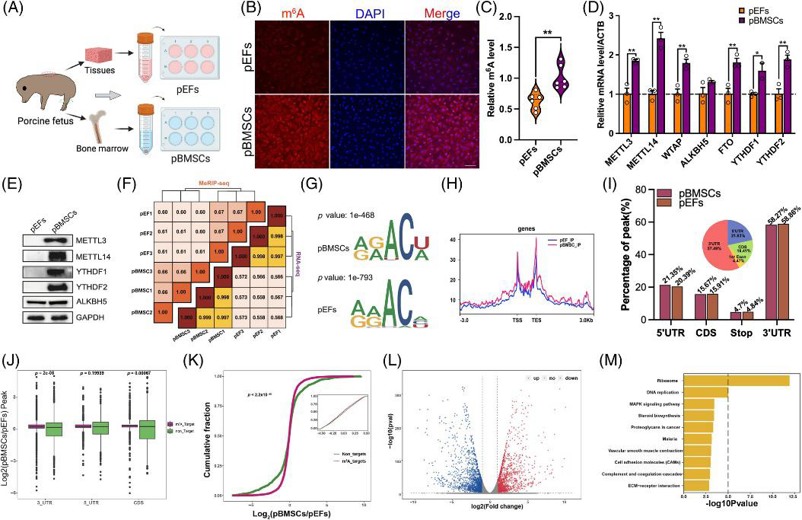
The Characteristics of RNA m6A Modification in pBMSCs and pEFs
Epigenetic reprogramming is essential for SCNT embryo development. The role of RNA N6-methyladenosine (m6A) in SCNT embryos has not yet been identified. Zhang's team investigated how RNA m6A affects the developmental competency of SCNT embryos derived from porcine bone marrow mesenchymal stem cells (pBMSCs) and porcine embryonic fibroblasts (pEFs).
The researchers compared RNA m6A levels between pBMSCs and pEFs by isolating and culturing these cells. Immunofluorescence and ELISA indicated increased RNA m6A levels in pBMSCs compared with those in pEFs. qPCR and Western blotting showed that the genes involved in RNA m6A methylation were upregulated in pBMSCs (Fig. 1). The difference was further verified by MeRIP-seq. The RNA m6A level was strongly correlated with gene expression in the same cell type (Fig. 1F). The m6A consensus sequences were GGACU and AAACA for pBMSCs and pEFs, respectively (Fig. 1G), which was similar to the pig RNA m6A motif reported previously. RNA m6A distribution primarily occurs at transcription start sites and transcription end sites. The RNA m6A level was also higher in pBMSCs than that in pEFs (Fig. 1H). The majority of RNA m6A sites (~58%) were located in the 3' UTRs, followed by the 5' UTRs (~20%) (Fig. 1I). Genes with RNA m6A had lower mRNA levels in pBMSCs compared with those in pEFs (Fig. 1K). KEGG pathway analysis demonstrated that genes with differential methylation levels showed significant enrichment in pathways like Ribosome as well as Proteoglycans in cancer and MAPK signaling pathway among others (Fig. 1M). The results suggested that the difference in RNA m6A between pBMSCs and pEFs might affect SCNT embryo development.
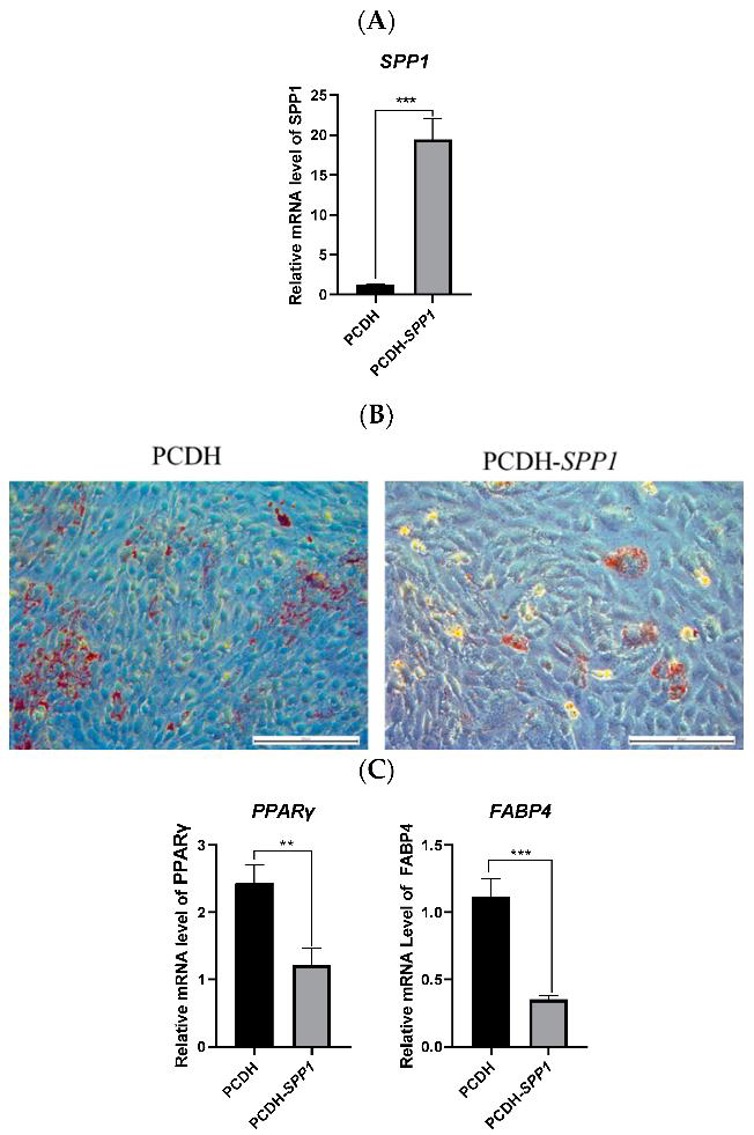
SPP1 Affected the Adipogenic Differentiation of pBMSCs
Castration in hog production eliminates boar taint but causes excessive fat accumulation. Xiao's team investigated the role of secreted phosphoprotein 1 (SPP1) in regulating adipose deposition in castrated pigs, using overexpression and interference in porcine bone marrow mesenchymal stem cells (pBMSCs).
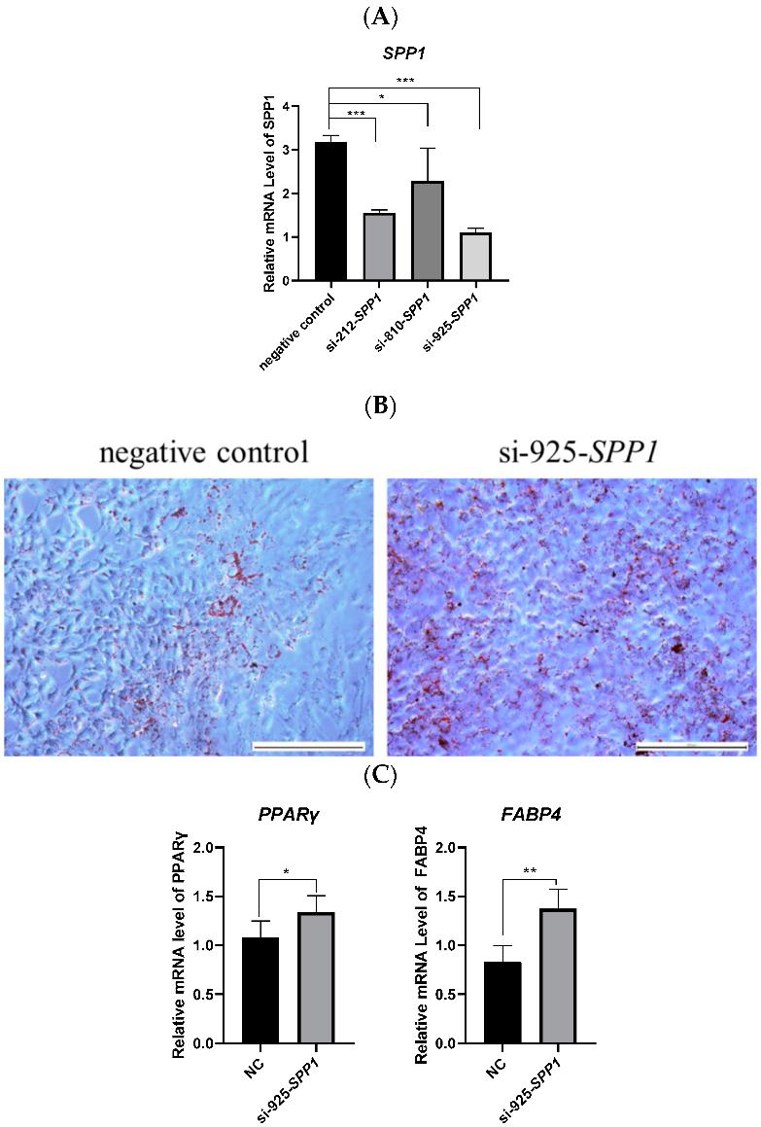
To study the role of the SPP1 gene in pBMSCs, they overexpressed it by transfecting PCDH-SPP1 into these cells (Fig. 2A). After 14 days of differentiation, cells with PCDH-SPP1 had fewer lipid droplets and lower levels of adipogenesis markers (PPARγ and FABP4) compared to controls (Fig. 2B, C). This shows that SPP1 overexpression reduces fat formation in pBMSCs. Next, they used three types of SPP1 siRNA to knock down SPP1 in pBMSCs. si-925-SPP1 worked best (Fig. 3A). After 14 days, cells treated with si-925-SPP1 had more lipid droplets and higher levels of PPARγ and FABP4 (Fig. 3B, C). This means that blocking SPP1 promotes fat formation in pBMSCs.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells