Hepatic Differentiation of Induced Pluripotent Stem Cells (iPSCs)
In vitro models of parenchymal liver cells are of great importance in toxicology and in bioartificial liver research, but primary cultures of hepatocytes have been hindered by their short life span and the rapid loss of hepatic functions under in vitro conditions. Therefore, it is necessary to find an alternative therapy to treat these serious liver injuries. Induced pluripotent stem cells (hiPSCs) as therapeutic tools as they can be obtained with relative ease and expanded in culture, along with features of self-renewal and multidirectional differentiation have attracted considerable attention.
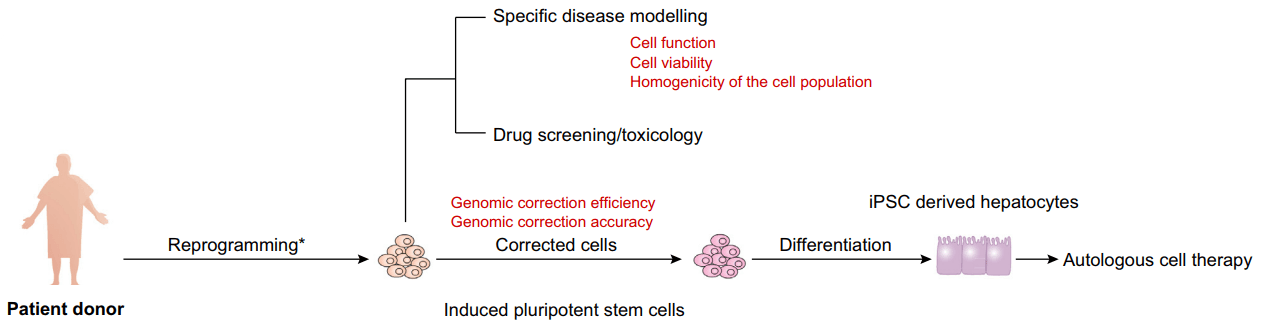
Figure 1. Applications of hiPSC-derived hepatocytes and their limitations (red) isolated from patients.
Materials
| Bone Morphogenetic Protein 4 (BMP4) | DPBS− with 0.02% EDTA, pH 7.4 |
| Fibroblast Growth Factor 2 (FGF2) | DPBS− (Ca2+/Mg2+ free DPBS) |
| Hepatocyte Growth Factor (HGF) | Pluripotent stem cell medium |
| Hepatocyte Culture Medium | B27 (with/without insulin) |
| RPMI-1640 medium | Accutase solution |
| Oncostatin-M | Activin A |
| Matrigel | Incubator |
Plate Cells for Differentiation
- Rinse the plate of pluripotent stem cells with sterile DPBS−, discard the rinse, then incubate the cells for 2 min with 3 mL of DPBS−/0.02% EDTA at room temperature. As soon as the cells begin to detach, removing DPBS−/0.02% EDTA and flood the plate with 6 mL of pluripotent stem cell medium.
Note: If the pluripotent stem cells are cultured on Matrigel or are difficult to dissociate, they can be removed by incubating at room temperature with 3 mL of Accutase solution. - Dissociate the cells into small clusters containing 3-6 cells by pipetting, collect cells by centrifugation at 200 g for 5 min, then suspend in pluripotent stem cell medium.
- Transfer the cell suspension to a 6-well tissue culture plate, which has previously been coated with Matrigel. Culture the cells overnight at 37°C, 4% O2/5% CO2.
Note: The number of cells that produce the optimal differentiation varies by cell line and should be determined empirically. After overnight culture, cells should form a monolayer covering 80-100 % of the surface of the dish. The cell density at the onset of differentiation has a dramatic impact on differentiation efficiency and may need to be determined empirically for each cell line.
Induce Differentiation of the Cells
- Days 1-2
Replace the culture medium with RPMI medium that has been pre-warmed to 37°C supplemented with 2% B27 (without insulin), 100 ng/mL Activin A, 10 ng/mL BMP4, and 20 ng/mL FGF2 and culture with daily medium changes for 2 days at 37°C in ambient O2/5% CO2.
Note: If Insulin free B27 is unavailable, 1 μM of the PI-3 kinase inhibitor LY294002 can be added as an alternative.
- Days 3-5
Change the culture medium to RPMI/2% B27 (without insulin) containing 100 ng/mL Activin A and continue to culture with daily medium changes for an additional 3 days at 37°C, ambient O2/5% CO2.
Note: At the end of this stage of the differentiation, it is crucial that > 90% of cells express proteins that are characteristic of the anterior definitive endoderm including CXCR4, FOXA2, SOX17, and GATA4. In addition, the presence of proteins associated with pluripotent cells such as OCT4 should be minimal if detected at all.
- Days 6-10
Induce hepatic differentiation by changing the medium to RPMI/2% B27 (with insulin) supplemented with 20 ng/mL BMP4 and 10 ng/mL FGF2 and continue to culture with daily medium changes for a total of 5 days at 37°C, 4% O2/5% CO2.
Note: After 5 days of culture, the cells should form a continuous monolayer and 80-90% of cells should express HNF4a and the levels of GATA4 and SOX17 should have declined.
- Days 11-15
Culture the hepatic progenitor cells for 5 days in RPMI/2% B27 (with insulin) supplemented with 20 ng/mL HGF with daily medium changes at 37°C, 4% O2/5% CO2.
Note: In addition to HNF4a, 80-90% of the cells should now express AFP and lipid droplets are commonly observed within the cytoplasm of the cells.
- Days 16-20
Replace the medium with Hepatocyte Culture Medium containing 20 ng/mL of Oncostatin-M. Continue to culture the cells for at least 5 days with daily medium changes at 37°C, ambient O2/5% CO2.
Note: By day 20 of the differentiation, the cells should display a morphology that resembles primary hepatocytes with a distinct cuboidal morphology and a large cytoplasmic to nuclear ratio. In addition, 70-90% of cells should express Albumin and the Asialoglycoprotein receptor (ASGPR).
References
- Mallanna S. K. et al. Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol, 2013, 26: 1G.4.1-1G.4.13.
- Hannoun Z. et al. The potential of induced pluripotent stem cell derived hepatocytes. Journal of Hepatology, 2016, 65: 182-199.