Reagent-based Transfection of hiPSCs to Deliver CRISPR-Cas9 Plasmids
This protocol uses the Lipofectamine Stem Transfection Reagent for efficient transfection of hiPSCs without the need for special instrumentation. It is appropriate for plasmid-based genome editing of hiPSCs cultured under xeno-free conditions on recombinant human laminin-521 in E8 medium, and can efficiently deliver the CRISPR-Cas9 plasmid (pX330) alone to induce insertions and deletions through NHEJ or with a pHDR construct to achieve HR-mediated modifications.
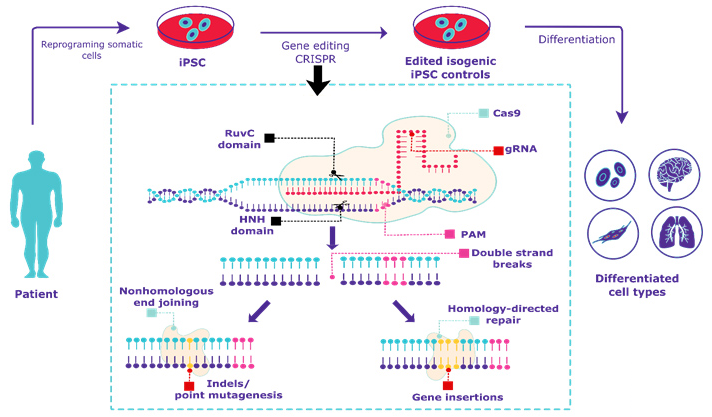 Figure 1. Overview of CRISPR gene editing of human iPSCs.
Figure 1. Overview of CRISPR gene editing of human iPSCs.
Materials and Equipment
| Recombinant human laminin-521 | Dulbecco's phosphate-buffered saline (DPBS) |
| Complete E8 medium (Essential 8 medium, 10 ng/mL rhFGF2, 100 μg/mL Primocin) | Opti-MEM I Reduced Serum Medium |
| 100X RevitaCell Supplement | Lipofectamine Stem Transfection Reagent |
| pX330 | Versene solution |
| NucleoSpin RNA kit | NucleoSpin Tissue kit |
| AccuPrime Taq DNA Polymerase, high fidelity (including 10X Buffer II) | NanoDrop One Microvolume UV-Vis Spectrophotometer |
| cDNA Synthesis Kit | MinElute PCR Purification Kit |
| E-Gel Agarose Gels Starter Pak, 2% | T7 Endonuclease I (including NEB Buffer 2) |
| Thermocycler | Cell counter or hemocytometer |
Perform Transfection
1) Prepare rhLaminin-521-coated plates and hiPSCs as described in Electroporation of hiPSCs to Deliver CRISPR-Cas9 Plasmids.
Note: Each transfection reaction will require one well of a 6-well plate of hiPSCs at 40%-50% confluency.
2) Prior to transfection, warm Opti-MEM I medium and Lipofectamine Stem Transfection Reagent to room temperature.
3) Pretreat hiPSCs with 1.5 mL/well of complete E8 medium containing 1X RevitaCell for 30 min in the 37°C incubator.
4) Aliquot the appropriate amount of plasmid into a 1.5 mL microcentrifuge tube. For gene disruption, use 2 μg pX330 per reaction. For gene correction, use 1.2 μg pX330 and 800 ng pHDR per reaction.
5) Add 50 μL Opti-MEM I per reaction to the plasmid(s).
6) Add 12.5 μL Lipofectamine Stem Transfection Reagent per reaction, then mix gently and incubate 10 min at room temperature.
7) Distribute the transfection complexes evenly to the wells of pretreated cells.
8) Incubate 48-72 h and observe cells for reporter expression.
Note: a) Removal of complexes by changing the medium after transfection is not required. b) Plasmid-based transfections require time for nuclear incorporation and expression.
Assessment of Genomic Modifications
Prepare gDNA and/or cDNA
1) Collect hiPSCs from wells of interest.
2) Isolate RNA or gDNA using the NucleoSpin RNA or NucleoSpin Tissue kit, respectively. Follow manufacturer's instructions with one modification in the first step for each protocol:
For RNA: Add 350 μL Buffer RA1 and 3.5 μL β-mercaptoethanol directly to each well.
For gDNA: Add 200 μL Buffer T1, 25 μL proteinase K solution, and 200 μL Buffer B3 directly to each well.
3) Determine the concentration and quality of each RNA or gDNA sample by spectrophotometry.
Note: Other methods of DNA quantification are also sufficient.
4) Generate cDNA from RNA using the cDNA Synthesis Kit according to manufacturer's instructions.
Perform PCR
1) Set up the reaction mix as follows (50 μL/reaction):
5 μL 10X AccuPrime Buffer II
100 ng gDNA or cDNA
2 μL target oligo F (20 nM)
2 μL target oligo R (20 nM)
0.25 μL AccuPrime Taq
ddH2O to 50 μL
Note: One primer must target a sequence that is not contained in the pHDR to avoid amplification of residual plasmid DNA. Repeat this process for the upstream junction. For RT-PCR, include amplifications of a housekeeping gene such as POL2RA as a control.
2) Perform amplification using the following parameters:
For gDNA:
34 cycles: 3 min at 94°C
15 ec at 94°C
20 ec at 58°C
1 min at 72°C
5 min at 72°C
Hold at 4°C
For cDNA: Increase the number of cycles to 40.
3) Check amplification by running 5 μL of each reaction on a 2% agarose gel.
Note: Any method of DNA electrophoresis can be used, such as the E-gel system. It is important that the PCR results in a single product of the correct size.
4) Column purify the remainder of each reaction using the Qiagen MinElute PCR Purification Kit according to manufacturer's instructions. Elute purified PCR product with 10 μL ddH2O.
5) Determine the concentration of the purified PCR products by spectrophotometry.
Note: At least 200 ng PCR product are required for heteroduplex formation and the T7 endonuclease I assay. For inefficient PCRs, perform replicates and purify them together.
Form heteroduplexes
1) Set up each reaction as follows:
2 μL 10X NEB Buffer 2
200 ng purified PCR product
ddH2O to 20 μL
2) Perform denaturation and slow annealing in a thermocycler using the following program:
95°C for 10 min
95-85°C, -2°C/sec then 85°C, 1 min
85-75°C, -2°C/sec then 75°C, 1 min
75-65°C, -2°C/sec then 65°C, 1 min
65-55°C, -2°C/sec then 55°C, 1 min
55-45°C, -2°C/sec then 45°C, 1 min
45-35°C, -2°C/sec then 35°C, 1 min
35-25°C, -2°C/sec then 25°C, 1 min
25-4°C, -2°C/sec then 4°C hold
Perform T7 endonuclease I assay
1) Add 1 μL T7 endonuclease I to each heteroduplexed reaction and incubate 30 min at 37°C.
Note: SNPs and sequences with secondary structures may result in false positives. Therefore, an untreated control is needed to validate the results.
2) Run entire reaction on a 2% agarose gel.
References
- Giacalone, J. C. et al. CRISPR-Cas9-based genome editing of human induced pluripotent stem cells. Current Protocols in Stem Cell Biology, 2018, 44: 5B.7.1-5B.7.22.
- Somali Chaterji, et al. CRISPR genome engineering for human pluripotent stem cell research. Theranostics, 2017, 7(18): 4445-4469.
- Byrne S. M. et al. Genome editing in human stem cells. Methods Enzymol, 2014, 546: 119-138.