General Immunostaining Procedure for Intracellular Antigens
The following protocol is intended for intracellular antigen analysis at the single cell level by flow cytometry. Typically, cells are fixed with formaldehyde to stabilize the cell membrane, then permeabilized with detergent or ethanol to allow antibodies against intracellular antigens, to access to stain intracellularly.
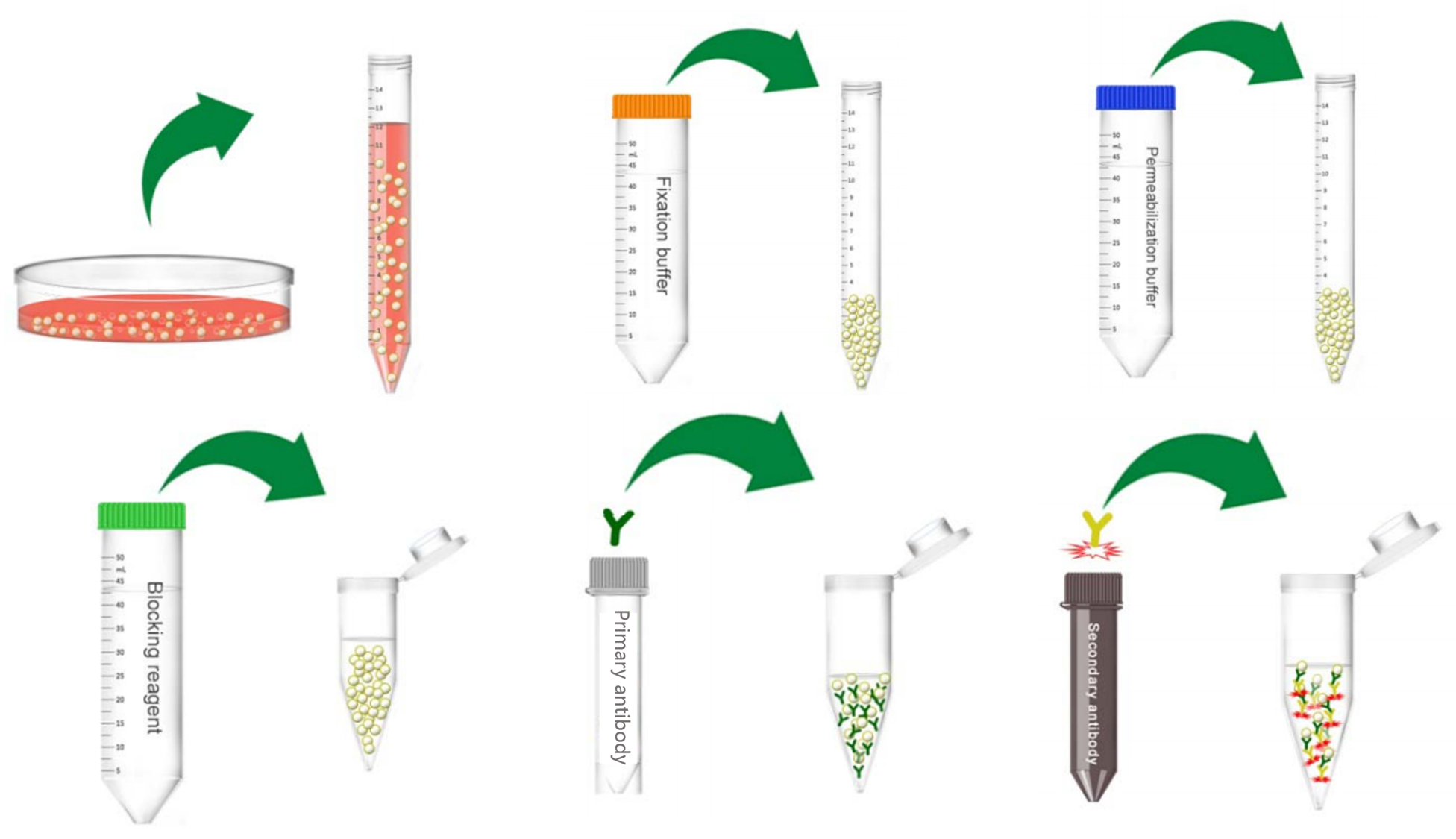 Figure 1. Schematic diagram of intracellular antigen staining.
Figure 1. Schematic diagram of intracellular antigen staining.
Materials
| 0.01% Formaldehyde | Permeabilization reagent |
| Primary antibody (fluorochrome/non-fluorochrome-conjugated) | Fluorochrome-conjugated second antibody |
| Refrigerated centrifuge | Phosphate buffered saline containing 1% bovine serum albumin (PBS/BSA) |
| Flow cytometer | Flow tube |
Cell Preparation
1) Collect the cells (more detail you can view Cell Preparation for Flow Cytometry), add PBS/BSA to resuspend the cells to a concentration of 1 x 107 cells/mL.
2) Aliquot 100 µL cell suspension (about 1 x 106 cells) and add it into the flow tube.
Fixation and Permeabilization
Fix cells before intracellular staining to ensure the stability of soluble antigens or antigens with a short half-life (see the special recommendations below for exceptions). This retains the target protein in the original cellular location.
Detection of intracellular antigens requires cell permeabilization before staining. Several methods are available for cell permeabilization:
1) Triton or NP-40 (0.1-1% in PBS) partially dissolve the nuclear membrane, so they are suitable for nuclear antigen staining. Loss of cell membrane and cytoplasm will result in decreased light scattering and reduced non-specific fluorescence.
2) Tween 20, Saponin, Digitonin and Leucoperm (0.5% v/v in PBS) enable antibodies to go through pores without dissolving plasma membrane. They are suitable for antigens in the cytoplasm or the cytoplasmic face of the plasma membrane and soluble nuclear antigens.
3) Nuclear targets are reached using methanol, or ethanol as the permeabilizing agent. Ethanol is preferred for the proliferation-specific target Ki-67 but methanol is more commonly used.
- Formaldehyde followed by permeabilizing reagent
1) If the cell surface staining is needed, follow the Cell Surface Immunophenotype Analysis protocol firstly.
2) Then add 100 uL of 0.01% formaldehyde to the tube and incubate for 10-15 min at room temperature.
3) Centrifuge at 300 g for 5 min and discard the supernatant.
4) Add 1 mL of permeabilizing reagent and incubate for 10 min at 4°C, protecting from light.
5) Centrifuge at 300 g for 5 min and remove the supernatant.
- Special recommendations
1) Antigens close to the plasma membrane and soluble cytoplasmic antigens will require mild cell permeabilization without fixation.
2) Cytoskeletal, viral and some enzyme antigens usually give the optimal results when fixed with a high concentration of acetone, alcohol or formaldehyde.
3) Antigens in cytoplasmic organelles and granules will require a method of fixation and permeabilization depending on the antigen.
4) When gating on cell populations, the light scatter profiles of the cells will change considerably after permeabilization.
Direct Staining Procedure
1) Add 50 µL of permeabilizing reagent and the appropriate amount of the fluorochrome-conjugated antibody.
2) Vortex briefly and incubate for 30 min at 4°C in the dark.
3) Wash twice with 1 mL of PBS/BSA by centrifugation at 300 g for 5 min.
4) Resuspend samples in 0.5 mL of PBS/BSA and hold them at 4°C protected from light prior to analysis.
Indirect Staining Procedure
1) Process the samples as above using a working dilution of un-labelled antibody.
2) Resuspend cell pellet and then add 50 µL of a working dilution of the fluorochrome-conjugated second antibody.
3) Vortex briefly and incubate for 20 min at 4°C in the dark.
4) Wash twice with 1 mL of PBS/BSA by centrifugation at 300 g for 5 min.
5) Resuspend samples in 0.5 mL of PBS/BSA and hold them at 4°C protected from light prior to analysis.
Note
1) Always use isotypic controls of the same heavy chain class at the same protein concentration as your relevant antibody for determination of background staining.
2) For storage of samples longer than overnight and for biohazard considerations, the samples can be re-suspended in 1% formaldehyde solution after the internal staining procedure has been completed.
Troubleshooting
Whenever the results are not satisfactory, please consider the following: 1) the precise optimal conditions for staining of intracellular antigens may be influenced by the nature of the antigen and its localization. It might be necessary to modify the length of the incubation with the antibody and/or the temperature of the reaction. 2) Keeping the antibody in the detergent solution during the incubation step has also been described as a measure to improve penetration of the reagent to the reaction site. 3) In addition, it is also known that some cytoplasmic or nuclear antigens require a higher degree of fixation and/or a different fixative. Therefore, modification of the concentration of the fixative and/or the temperature of the fixation step can improve staining of some internal proteins.
References
- Turaç G. et al.; Combined flow cytometric analysis of surface and intracellular antigens reveals surface molecule markers of human neuropoiesis. PLoS ONE, 2013, 8(6): e68519.
- Bardales R. et al.; Detection of intracytoplasmic immunoglobulin by flow cytometry in B-cell malignancies. J Histochem Cytochem, 1989, 37: 83-89.
Cell Services:
Cell Line Testing and Assays: