Cell Surface Immunophenotype Analysis
Cell surface markers can be used to define cell subsets based on lineage and developmental stage, as well as function when they are labeled with fluorochrome-conjugated antibodies and analyzed by flow cytometry. These surface markers have different forms and functions including receptors for both soluble and cell-bound ligands, ion channels, glycoproteins, phospholipids, and more. For example, CD4 is a surface marker for T helper cells that can be further differentiated based on expression of other chemokine receptors and cluster of differentiation (CD) markers. Live cells stained with antibodies can be sorted according to unique staining patterns and used for additional experiments.
This method provides a general procedure for cell surface immunophenotype analysis. A certain level of technical skills and immunological knowledge are required for the successful design and implementation of these techniques, the following are guidelines only and may need to be adjusted and optimized for particular applications.
Materials
- Phosphate buffered saline containing 1% bovine serum albumin (PBS/BSA)
- Red blood cell lysing buffer
- Anticoagulant (For basic staining, any appropriate anticoagulant such as heparin, EDTA, or acid citrate dextrose, may be used. In some instances, specific anticoagulants may be required)
- Primary antibodies
- Secondary reagents, if necessary (for indirect staining)
- Human Fc receptor blocking solution
Assay Procedure
Harvest cells
- Prepare a single cell suspension as described in “Cell Preparation for Flow Cytometry” for further information. Adjust the cell suspension to a concentration of 1 x 107 cells/mL with cold PBS/BSA.
Note: Whole blood samples may be used undiluted unless the cell count is high, e.g. as in leukemia. Use appropriate anticoagulants. - Aliquot 100 μL of the cell suspension or whole blood into as many test tubes as required.
Note: To the blood suspension, add 2 mL freshly prepared red cell lysing buffer and mix well. Incubate for 10 minutes at room temperature. Centrifuge at 300-400 g for 5 minutes at room temperature and discard the supernatant. Wash with 2 mL room temperature PBS/BSA, centrifuge at 300-400 g for 5 minutes and discard the supernatant.
Block Fc-receptors
Reagents that block Fc receptors may be useful for reducing nonspecific immunofluorescent staining.
- In humans, cells can be pre-incubated with 10 µL of Fc receptor blocking solution per 100 µL of cell suspension for 5-10 minutes at room temperature.
- In the absence of an effective/available blocking antibody, an alternative approach is to pre-block cells with excess irrelevant purified Ig from the same species and same isotype as the antibodies used for immunofluorescent staining.
Cell surface staining with antibody
- Add appropriately conjugated fluorescent, biotinylated, or purified primary antibodies at predetermined optimum concentrations (e.g. anti-CD3-FITC, anti-CD4-Biotin, and anti-CD8-APC) and incubate for 15-20 minutes in the dark.
- Wash cells with at least 2 mL of PBS/BSA and centrifuge at 300-400 g for 5 minutes.
- If a second-step reagent is required for detection of purified or biotinylated primary antibody, add 100 μL appropriately diluted secondary reagent to the dissociated cell pellet and incubate in the dark for 20-60 minutes.
- Wash cells with at least 2 mL of PBS/BSA and centrifuge at 300-400 g for 5 minutes.
- Resuspend cells in 200 μL cold PBS or with 200 μL of 0.5% paraformaldehyde in PBS if required.
- Acquire data by flow cytometry. Analyze fixed cells within 24 hours.
Notes
- If you are unable to immediately read your samples on a cytometer, keep them shielded from light and in a refrigerator set at 4-8°C. Note that samples should not remain in a fixation buffer for extended periods of time as this can affect fluor conformation and fluorescence.
- Antibody-binding kinetics are temperature-dependent. Staining on ice may require longer incubation times. Furthermore, some antibodies may require non-standard incubation conditions that will be noted on the technical data sheet provided with the antibody.
- Appropriate controls should always be carried out. For flow cytometry, the following should be considered for inclusion: 1) isotype controls used to determine if the staining is specific; 2) unstained cells should always be included in the experimental set-up to monitor auto-fluorescence.
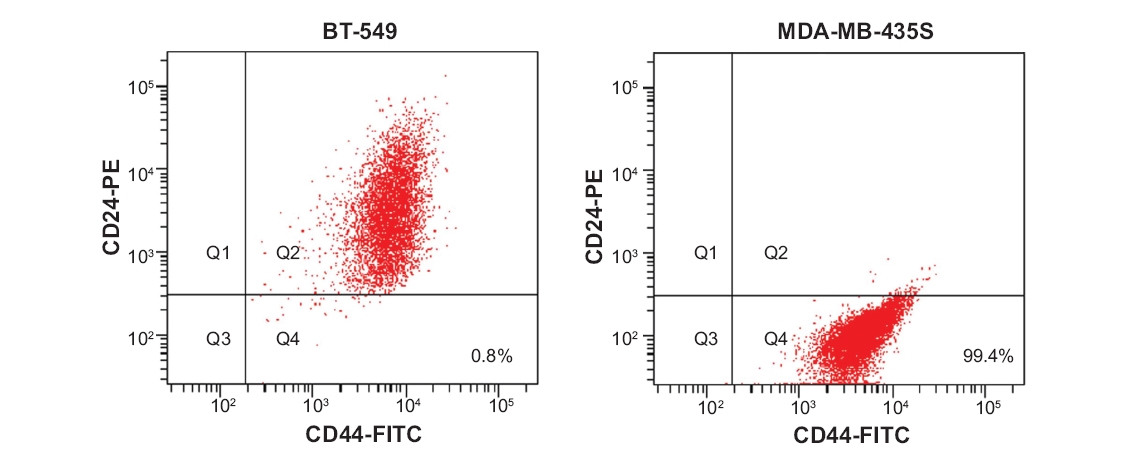 Figure 1. Identification of a CD44+/CD24- subpopulation in breast cancer cell lines.
Figure 1. Identification of a CD44+/CD24- subpopulation in breast cancer cell lines.
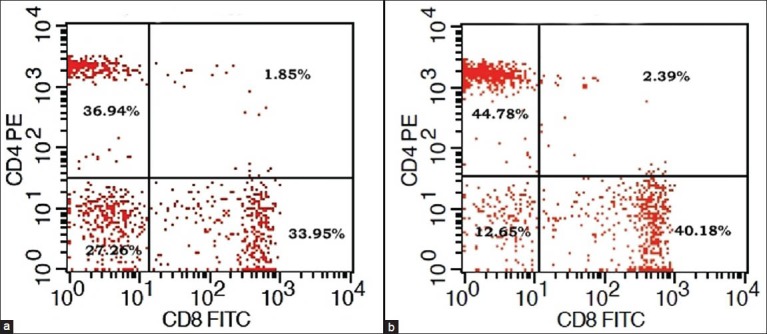 Figure 2. CD4+/CD8+ percentage of T-lymphocytes in a patient.
Figure 2. CD4+/CD8+ percentage of T-lymphocytes in a patient.
Cell Services:
Cell Line Testing and Assays: