The Syrian Hamster Embryos (SHE) Cell Transformation Assay
Since testing a carcinogenic substance in experimental animals is very expensive, it has become necessary to develop cheaper and faster in vitro assays to assess the potential carcinogenicity of chemicals. The assays can be divided into two types depending on the cells used. The first type of cell transformation assay uses established cell lines that lack one or more cancer-related endpoints, and the acquisition of various cancer-related properties are measured. The second, perhaps better system, uses primary cell lines. These cells have a limited life span during culture, are not immortalized, and are useful for studying the acquisition of a number of cancerous properties following exposure to carcinogens.
The most common properties that have been used to examine cell transformation of primary cultures are the growth pattern and morphological transformation of the cells. Cells are exposed to a test substance, plated to form colonies, and the growth properties of the colonies after treatment are studied. The establishment of primary cultures from Syrian hamsters is preferred over other rodents because they have much lower rates of spontaneous immortalization. In addition, SHE cells have been widely used in carcinogenesis test therefore data is available for a large number of test agents.
Materials
| Serum-free Dulbecco’s minimal essential medium (DMEM) | Complete DMEM supplemented with 10% (v/v) FBS |
| F-12 medium supplemented with 10% (v/v) FBS | Disinfectant solution (e.g., 2% (v/v) orsyl) |
| Pregnant golden Syrian hamsters | Chinese hamster ovary (CHO) |
| 0.5% (w/v) trypsin solution in normal saline A | 0.5% (w/v) crystal violet solution in 95% ethanol |
| Fetal bovine serum (FBS) | Serum-free F-12 medium |
| Absorbant cloth, sterile | Laminar flow hood |
| Dissecting instruments | 95% (v/v) ethanol |
| 10 mL pipets, sterile | 150 mL beakers |
| 100 mm tissue culture plates, sterile | Normal saline A (3.3 mM NaHCO3, 5 mM KCl, 130 mM NaCl, 5 mM glucose, filter sterilize) |
| Inverted tissue culture microscope | Coulter counter or hemacytometer |
Assay Procedure
Harvest hamster embryos
- Obtain pregnant golden Syrian hamsters on day 13 of gestation.
Note: 1) The Syrian hamster embryo system is the preferred system because it has been claimed that these cells have a low incidence of spontaneous transformation. 2) The 13th day of gestation is the optimal time to harvest embryos, however, cells from 12-or 14-day-old embryos can also be isolated for tissue culture. 3) Pregnant hamsters can be obtained from commercial sources. Alternatively, they can be bred, but this is not recommended unless one has experience with breeding hamsters. - Sacrifice the hamster by CO2 asphyxiation and immediately immerse in a disinfectant solution for a few seconds.
- Place the animal on an absorbent sterile cloth in a sterile laminar flow hood.
- Sterilize the dissection tools by dipping them in a 150 mL beaker containing 95% ethanol.
- Lay the hamster on its dorsal side and make an incision in the uterus to expose the embryonic sacs.
- Using forceps and scissors, carefully remove the embryonic sacs and place them into a sterile 100 mm tissue culture dish.
- Remove the embryo from each sac, and use the alive ones (as gauged by body movement) for isolation of cells.
Isolate embryonic cells
- Wash the embryos twice with 10 mL serum-free DMEM.
- Transfer the embryos to a fresh 100 mm tissue culture dish containing 10 mL of 0.5% trypsin solution in normal saline A. Mince the embryos with sterile scissors.
- Disperse into individual cells by repeatedly passing the mixture up and down through a 10 mL sterile pipet. Incubate the cells in the 0.5% trypsin solution for about 30 min.
- Terminate the action of trypsin by adding 1 mL FBS.
- Seed 5 x 106 to 1 x 107 cells in 10 mL of complete DMEM-10% FBS into 100 mm tissue culture plates.
Note: Cellular transformation levels can vary greatly depending upon the source and/or lot of FBS.
Primary cell culture
- Place the plates in a 37°C incubator with an atmosphere containing 5% CO2. Incubate for 2 days.
- Wash the monolayer twice with 10 mL sterile normal saline A and disrupt the monolayer by adding 10 mL of 0.5% trypsin solution in normal saline A. Centrifuge the cells for 10 minutes at 500 g. Aspirate the supernatant and resuspend the cell pellet in 10 mL of fresh complete DMEM-10% FBS.
- Replate at a density of 5 x 106 cells/100 mm plate in 10 mL complete DMEM-10% FBS.
Determine cytotoxicity of test agent
- Plate 1 x 106 CHO cells in 10 mL complete F12-10% FBS and allow to attach overnight.
Note: The plating efficiency of primary cultures is so low that they are not optimal for determining cytotoxicity. Thus, an established cell line of hamster cells (e.g., CHO cells) should be used to obtain an approximate level of cytotoxicity of the chemical. - Expose these cells to the test agent using a broad range of dosages (e.g., 0.1, 0.2, 0.5, 1.0, 5, and 10 μg/mL) in order to establish an appropriate dosing regimen for the cell transformation assay.
- Following treatment, remove the chemicals from the culture plates and wash cells twice with normal saline A.
Note: The medium may contain toxic and/or carcinogenic chemicals and should be disposed of in an appropriate manner. - Trypsinize the monolayer with 0.5% trypsin and count cells with a coulter counter.
- Determine cell viability by trypan blue exclusion using 0.4% (w/v) trypan or hemacytometer blue solution.
- Plate 100 to 1,000 cells into 100 mm tissue culture plates in 10 mL complete DMEM-10% FBS.
- Incubate for 2 weeks at 37°C in 5% CO2. Change the medium twice weekly during this period.
- Wash the cells with normal saline A and fix in 95% ethanol for 5 min. Stain with 0.5% crystal violet solution in 95% ethanol for 5 min.
- Wash the excess stain and count the number of colonies and calculate the plating efficiency.
Plating efficiency = (number of colonies/number of cells plated) x 100
Note: 1) Plating efficiency refers to the percentage of cells that are initially plated that give rise to colonies. This information is used in the cell transformation assay to determine the starting number of cells to plate in order to obtain a sufficiently large number of surviving colonies per dish. 2) It should be noted that the cytotoxicity in this particular cell line may not be exactly the same as that in SHE cells; therefore, adjustment to the dose is sometimes needed. Generally, a dose that is toxic to one hamster cell line will also be toxic to another hamster cell line.
Determine cell transformation
- Trypsinize unexposed SHE cells and neutralize the trypsin with an equal volume of fresh complete DMEM-10% FBS.
- Seed 1,000 to 5,000 cells into 100 mm tissue culture plates and allow to attach for 1 or 2 days. Treat with the test compound or a positive or negative control.
Note: Cell numbers may vary depending upon the experiment. At higher exposure levels, 5,000 to 20,000 cells may be plated to counteract the low plating efficiency of these cells. - Place cells in a 37°C, 5% CO2 humidified incubator for 2 weeks or until visible colonies are formed. Replenish the medium twice weekly during this incubation period.
Note: Care must be taken not to shake or agitate the plates during this procedure, because this may dislodge cells from colonies. This would result in the formation of new colonies, artificially increasing or decreasing the apparent incidence of transformation by giving birth to more than one colony from one cell at risk. - Aspirate the medium and wash cells twice with normal saline A. Fix with 95% ethanol for 5 min.
- Stain with 0.5% crystal violet solution in 95% ethanol for 5 min.
- Wash off excess stain.
Assess the degree of morphological transformation
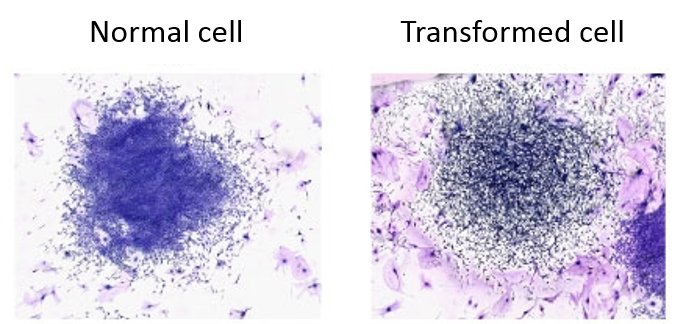
- Count the number of surviving colonies in each plate and examine them for morphological transformation. Observe each colony under an inverted tissue culture microscope for cell crisscrossing and overlapping.
- Calculate the morphological transformation frequency as the (number of transformed colonies/total number of colonies) x 100.
References
- Berwald Y, et al.; In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst, 1965, 35: 641-661.
- Dunkel V C. et al.; Recommended protocols based on a survey of current practice in genotoxicity testing laboratories: III. Cell transformation in C3H/10T1/2 mouse embryo cell, BALB/c3T3 mouse fibroblast and Syrian hamster embryo cell cultures. Mutat. Res. 1991, 246: 285-300.
Cell Services:
Cell Line Testing and Assays