Tumorsphere Formation Assay
Tumorsphere is a solid, spherical structure developed from the proliferation of the cancer stem/progenitor cells. These tumorspheres are easily distinguished from single or aggregated cells as these cells appear to fuse together and individual cells do not. Tumorsphere cultivation refers to plating cancer cells onto the ultralow attachment surface in serum-free media supplemented with growth factors such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). Tumorsphere cultivation is widely used to analyze the self-renewal ability of CSCs and to enrich these cells from bulk cancer cells. This method also provides a reliable platform for screening potential anti-CSC agents.
Compared with general monolayer culture, the in vitro anti-proliferation activity of potential agents selected from tumorsphere assay is more likely to be converted into in vivo anti-tumorigenic activity. Tumorsphere assay can also measure the outcome of clinical trials for potential anti-cancer agents. In addition, tumorsphere assay may be a promising strategy in the innovation of future cancer treatments and may help in the screening of small molecule chemicals.
Materials
| Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12) | Sterile phosphate buffered saline |
| Basic fibroblast growth factor (bFGF) | Epidermal growth factor (EGF) |
| Bovine serum albumin (BSA) | B27 supplement |
| Trypan blue | Insulin |
Reagents Preparation
Tumorsphere medium (20 ng/mL EGF,10 ng/mL bFGF, 5 μg/mL insulin, 0.4% BSA, 500 mL DMEM/F12)
Assay Procedure
1) Add B27 supplement to tumorsphere medium as needed for experiments. B27 should be freshly added before each experiment.
2) Harvest cells and suspend the resulting pellet in 5 mL of sterile PBS. Keep cells on ice while not in use for the duration of the experiment.
3) Gently mix the suspension and pipette 20 μL of the suspension into a tube.
4) Add 20 μL of trypan blue to the cells in the tube and mix well.
5) Pipette approximately 10 μL of the mixture onto the hemocytometer.
6) Count only the bright cells within the four corner quadrants of the counting chamber for viable cell count.
7) Take the needed number of cells and add the appropriate volume of tumorsphere medium to make the cell concentration at 1 cell/μL. Keep this suspension on ice and mix well for plating.
8) Add PBS to the first and last columns (column 1 and 12) of the 96-well plate to help minimize medium evaporation. This will leave 10 wells available for each row.
9) Seed 200 μL of the cells suspended in tumorsphere medium into each well (200 cells per well).
10) For each cell line or treatment, seed cells into the wells of 2 rows for a total of 20 wells. This will equal a total of 4,000 cells.
11) Seal the upper and lower edges of the 96-well plate with laboratory tape to avoid evaporation of medium and place the cells in an incubator set to 37°C and supply the cells with 5% CO2 for one week.
12) After one-week incubation, tumorsphere numbers are counted under a phase-contrast microscope using the 40X magnification lens.
13) Results can be presented as a percentage of the number of tumorspheres present divided by the initial number of cells seeded (4,000 cells).
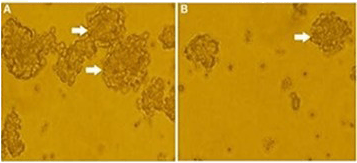 Figure 1. Tumorspheres formed by MDA-MB-231 cells.
Figure 1. Tumorspheres formed by MDA-MB-231 cells.
Note
1) The addition of B27 has been shown to increase tumorsphere formation and maintain several passages of sphere cultures.
2) The number of cells used for tumorsphere formation assays may vary with the cell type.
3) Serum-free media can sometimes lead to cellular death. It may be beneficial to supplement with 1% FBS to prevent death. Alternatively, you can use low-serum media in the absence of additional growth factor supplementation.
4) Be sure to allow enough time for spheres to form. This may take up to one week, based on the doubling time of different cell types.
5) The morphology of spheres may vary depending on the cell line or primary tissue used. Some spheres may be more compact while others may be loose.
References
- Johnson S. et al. In vitro tumorsphere formation assays. Bio Protoc, 2013, 3(3): e325.
- Patel S. et al. Tumorsphere passage for breast cancer stem cells. Protocol Exchange, 2013.