The BALB/c 3T3 Cell Transformation Assay
In vitro mammalian cell transformation assays have been widely used to test chemical and physical agents for the ability to induce morphological transformation. Morphologically transformed cells are characterized by the loss of density-dependent regulation of growth and the formation of colonies with crisscrossed cells or foci of piled-up cells that are not observed in untreated controls. Because of the biological similarities between in vitro cell transformation and in vivo carcinogenesis, cell transformation assays are used routinely to screen chemicals for carcinogenic potential and to study the mechanisms of known carcinogens.
The in vitro cell transformation assay using the BALB/c 3T3 cell line is based on the change of the phenotypic features of cells undergoing the first steps of the conversion from normal cells to neoplastic-like cell foci with oncogenic properties. Carcinogenesis is a complex multistage process by which normal cells are transformed into cancer cells characterized by an accumulation of changes at the cellular, genetic and epigenetic level. The BALB/c 3T3 cell transformation assay has been shown to recapitulate a multistage process that closely models some stages of in vivo carcinogenesis.
Materials
- Balb/c 3T3 mouse fibroblast cell line (subclone A31-1-1)
- Fetal bovine serum (FBS)
- M10F medium (Minimum essential medium (MEM), FBS 10% (v/v), penicillin (10,000 units/mL), streptomycin (10 mg/mL))
- DF2I2F medium (Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12), FBS 2% (v/v), penicillin (10,000 units/mL), streptomycin (10 mg/mL), insulin stock solution (2 mg/mL))
Reagents
- EDTA-2Na solution
This 2% (w/v) solution is prepared by dissolving 2 g of EDTA-2Na in 100 mL of distilled or MilliQ water. The solution should be sterilized and can be stored at room temperature. - Washing solution
This solution consists of Ca2+/Mg2+-free phosphate buffered saline (PBS) containing 0.02% (w/v) of EDTA-2Na. It is prepared by adding 5 mL of 2% EDTA-2Na solution to 500 mL of Ca2+/Mg2+-free PBS. It can be stored at room temperature. - Trypsin solution
The 0.25% (w/v) trypsin solution is obtained from a commercial source and is stored at -20°C. - Fixing solutions
Methanol is used for cell fixation in the colony forming efficiency test and in the transformation assay. - Staining solutions
A 0.04% (w/v) Giemsa solution is used for cell staining in the colony forming efficiency test and the transformation assay. - Test substance solution
Test substances are dissolved or suspended in an appropriate vehicle and diluted with M10F complete medium. The preferred vehicle is DMSO. In general, the vehicle should not interact with the test substances nor affect cell survival or focus formation. The final vehicle concentration in the medium should be<10% (v/v) when the vehicle is distilled water or saline, and <0.1% (v/v) when the vehicle is DMSO, acetone or ethanol (however, a maximum concentration of 0.5% (v/v) of DMSO and acetone is acceptable in the medium when test substances do not dissolve at lower concentrations of these two organic vehicles).
Experimental Procedure
Dose range finding (DRF) experiment
Doses for the morphological transformation experiment are selected based on the results of the preliminary cytotoxicity tests: colony forming efficiency (CFE) and/or crystal violet (CV) experiments. CFE method is described hereafter.
- Cells at approximately 70% of confluence are trypsinized and suspended in M10F complete medium. Cells are then seeded in 60 mm dishes at a density of 200 cells/dish, in 4 mL of M10F complete medium (4 dishes/dose + 4 dishes for each of the controls: medium control (medium alone) and vehicle control (medium added with vehicle)).
- Cell treatment (24 h after seeding). Culture medium is replaced by 4 mL of treatment medium.
Note: 24 hours after seeding is the time necessary for the cells to exit the stationary phase of cell growth. Treatment media containing the various concentrations of test substances are prepared and used for medium change. - End of treatment (72 h after exposure). Treatment medium is replaced by 4 mL of fresh complete medium.
- Cell fixation and staining. After culture medium has been removed, cells are washed with saline and fixed with methanol (about 3 mL/dish) for 10 min. Then methanol is removed and cells are stained with a Giemsa solution for 30 min. After the staining solution is removed, the dishes are air-dried.
- Colony count. The colonies that have been grown are counted under a stereomicroscope. Only colonies with a well-defined center and more than about 50 cells/colony or more than about 2 mm in diameter are scored.
- Relative CFE and plating efficiency (PE) are recorded, and the results are expressed as relative mean CFE (%) ± SD.
Relative CFE (%) = total number of colonies formed in the treatment dishes x 100/total number of colonies formed in the control dishes.
PE (%) = number of colonies formed in the control dishes x 100/200, where 200 is the total number of cells seeded in the CFE dishes. - For the selection of doses to be used in the morphological transformation assay (MTA), the results of the DRF tests should contain at least one dose between the NOEL (No Observable Effect Level) and IC50 and another one between IC50 and IC90, as presented in Figure 1.
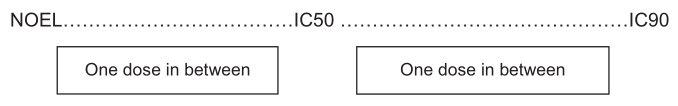 Figure 1. Doses required in the preliminary dose range finding tests for selecting the doses to be used in the MTA.
Figure 1. Doses required in the preliminary dose range finding tests for selecting the doses to be used in the MTA.
Morphological transformation assay (MTA)
- Day 0: Cells at approximately 70% of confluence are trypsinized and suspended in M10F complete medium. Cells are then seeded in each 100-mm dish at a density of 2 x 104 cells/dish.
- Day 1: The culture medium is removed from the dishes and 10 mL of the treatment medium are added to each of the corresponding dishes. Cells are exposed to the treatment medium for 72 hours.
- Day 4: Treatment medium is replaced with 10 mL of fresh M10F complete medium.
- Day 7 through Day 24 or 25: M10F medium is replaced with 10 mL of fresh DF2I2F complete medium at Day 7. Thereafter DF2I2F medium is changed twice a week.
- Day 31 or 32: Cells are fixed with methanol for 10 min and stained with a Giemsa solution for 30 min. After the staining solution is removed, dishes are air-dried.
- Focus count: For the analysis of the MTA, foci consisting of more than about 50 cells or being more than about 2 mm in diameter are evaluated using a stereomicroscope.
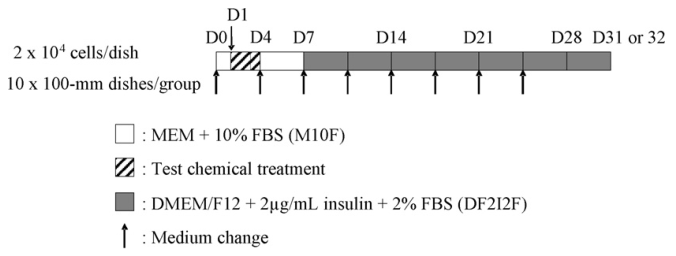 Figure 2. The workflow of the BALB/c 3T3 cell transformation assay.
Figure 2. The workflow of the BALB/c 3T3 cell transformation assay.
References
- Tanaka N. et al.; Prevalidation study of the BALB/c 3T3 cell transformation assay for assessment of carcinogenic potential of chemicals. Mutat Res. 2012, 744: 20-29.
- Schechtman L M. BALB/c 3T3 cell transformation: protocols, problems and improvement. IARC Sci. Publ. 1985, 67: 165-184.
Cell Services:
Cell Line Testing and Assays