Karyotype Analysis
The karyotype examines a person's chromosomes to determine if the right number is present and to determine if each chromosome appears normal. It requires experience and expertise to execute properly and to interpret the results. Karyotype analysis can be performed on almost any rapidly dividing cell population, whether it is grown in tissue culture or extracted from tumors.
The cultured cells are treated with colcemid, a drug that destroys the mitotic spindle to prevent the completion of mitosis and arrest the cells in metaphase. The harvested cells are treated briefly with a hypotonic solution that causes the nucleus to swell, making it easier for the technicians to identify each chromosome. The cells are then fixed, dropped on a microscope slide, dried, and stained. The most commonly used stain is the Giemsa stain, other dyes, such as fluorescent dyes, can also be used to produce banding patterns.
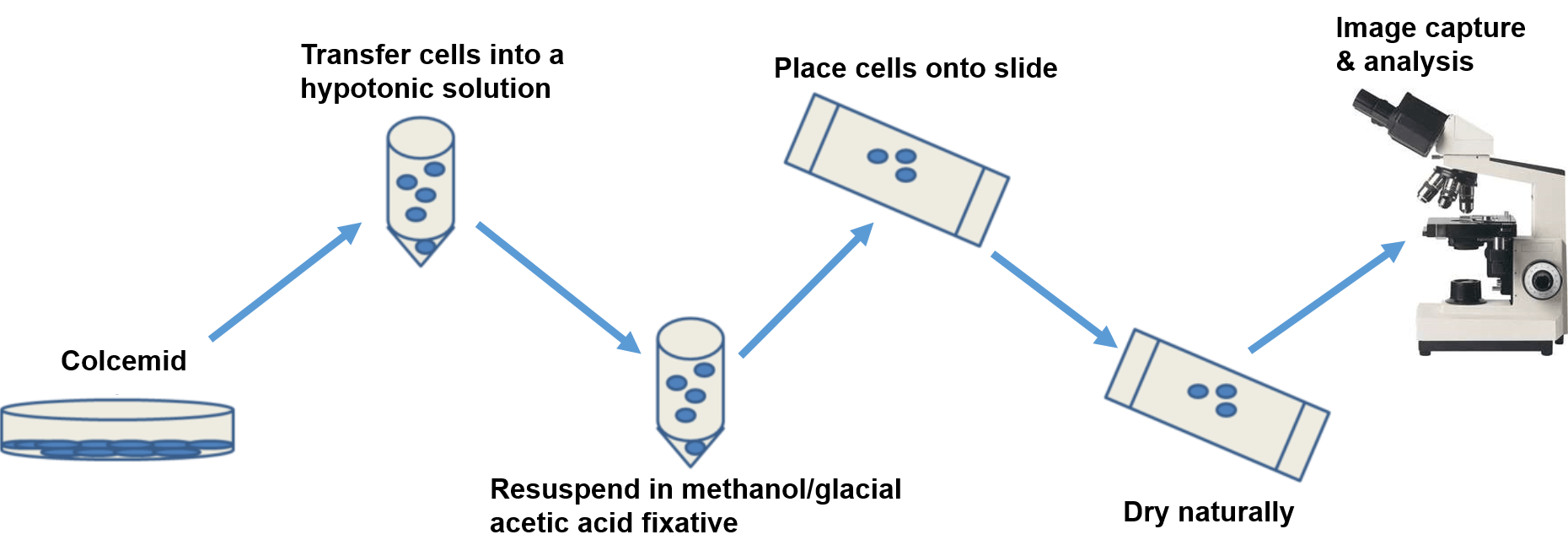 Figure 1. The basic workflow of karyotype analysis.
Figure 1. The basic workflow of karyotype analysis.
Materials and Equipment
| 1% Penicillin-streptomycin (10,000 U/mL; 10,000 μg/mL) | Hypotonic solution (0.075 M KCl) |
| 10% bfetal bovine serum (FBS) | Colcemid (10 μg/mL) |
| Glacial acetic acid | Cell lines: FaDu |
| CO2 incubator | Microscope |
| Trypsine |
Reagents Preparation
1) Fixative - Methanol and glacial acetic acid (3:1) to be made fresh and chilled before using
2) Growth medium - RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin (10,000 U/mL; 10,000 μg/mL)
Assay Procedure
1) Seed 5 x 105 cells on 10 cm cell culture dishes for attachment.
2) Cells were incubated at 37°C, 5% CO2 for 48 h.
3) Add 0.1 mL colcemid, which can collapse mitotic spindles and prevent the completion of mitosis, to each dish and mix gently. Incubate at 37°C, 5% CO2 for 2 h.
4) Add 1 mL of 0.1% Trypsine to the dishes and incubate for 2 min at 37°C. Then, transfer the mixture (Trypsine and cells) into a centrifuge tube and mix with the medium previously added. Further, centrifuge at 100 rpm for 10 min at room temperature.
5) Discard the supernatant and leave 0.5 mL medium to mix the pellet gently.
6) Resuspend the pellet in 5-7 mL of hypotonic solution and mix thoroughly. Incubate in water bath at 37°C for 10 min.
7) Centrifuge at 100 rpm for 10 min at room temperature.
8) Discard the supernatant and leave 0.5 mL solution to mix the pellet gently.
9) Resuspend the pellet in 5 mL of cold fixative (drop by drop and flick the tube between drops to prevent cell clumping).
10) Put the tube on ice for at least 20 min.
11) Centrifuge at 100 rpm for 10 min at room temperature.
12) Discard the supernatant and add 3-5 mL cold fixative.
13) Repeat steps 11)-12) three times or until the supernatant is clear and the pellet become white.
14) After the final centrifugation, suspend the cells in a few drops of cold fixative to give a slightly opaque suspension.
15) Drop 1-2 drops onto a wet and clean slide (previously, the slide should be rinsed with 1-2 drops of cold fixative.)
16) Dry the slide naturally at room temperature.
17) Observe the chromosomes with the microscope and count the number of chromosomes about 50 cells.
Cell Services:
Histology Services: