Fluorescence Compensation
All fluorochromes have excitation spectrum and emission spectrum. Within a flow cytometer, the appropriate ranges of emission wavelengths are selected by bandpass filters. However, when emission spectra overlap, fluorescence from more than one fluorochrome may be detected. In order to correct this spectral overlap, a process of fluorescence compensation is proposed. Compensation refers to the process of correcting the spillover from our primary signal in each secondary channel. This ensures that the fluorescence detected in a particular detector comes from the fluorochrome being measured.
By using fluorophores that do not have overlapping emission spectra, you can avoid the need for compensation. Alternatively, you can use fluorophores that can only be activated by specific laser lines, but as you increase the number of fluorophores, this becomes practically impossible.
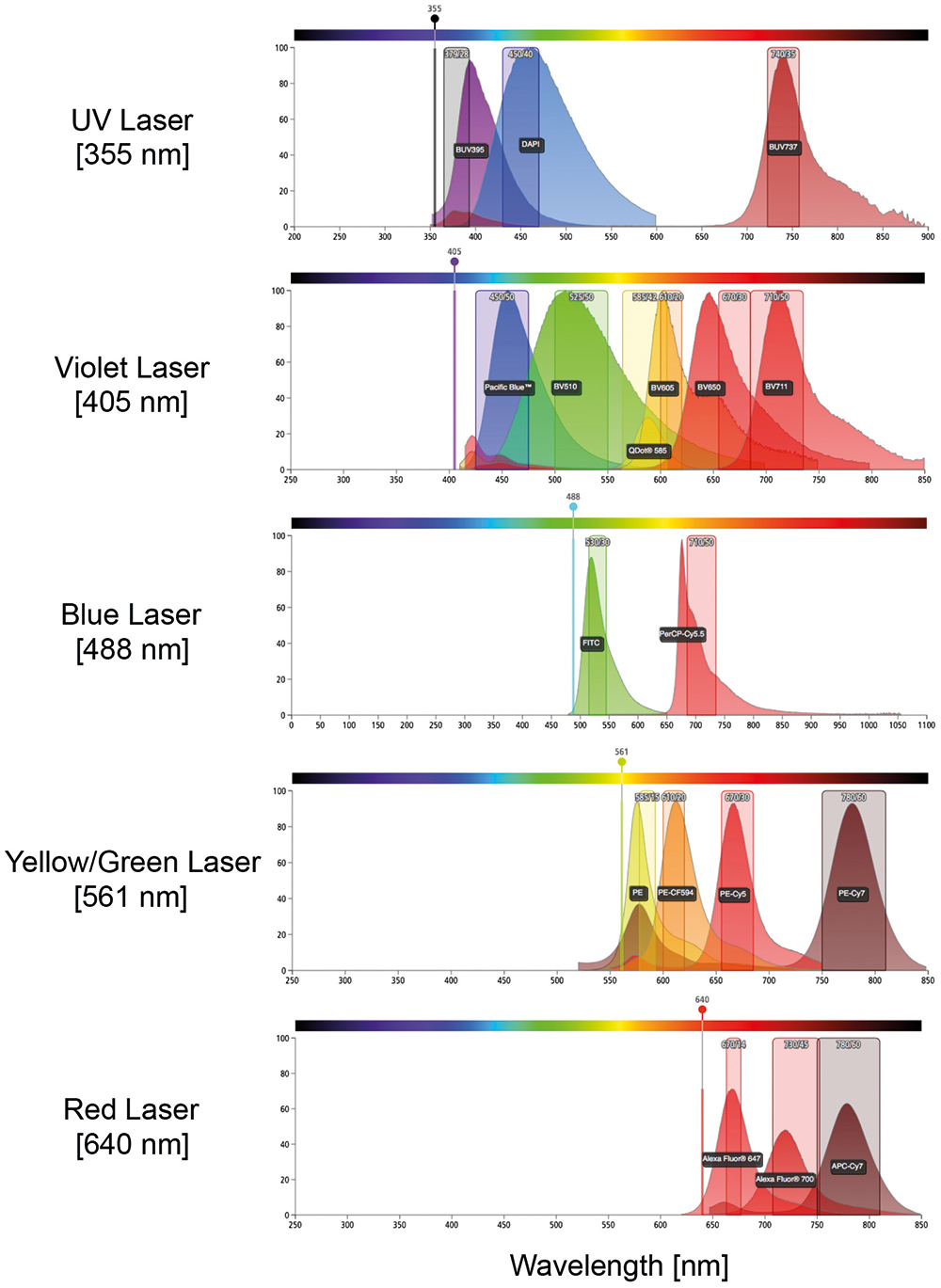 Figure 1. Schematic representation of diverse emission spectra.
Figure 1. Schematic representation of diverse emission spectra.
In some experiments, FITC may be combined with other dyes, for example PE, which emit yellow and orange photons. In this case, the relative contribution of each fluorophore to the signal must be determined. The emission spectrum of FITC and PE are shown below (Figure 2). Also shown is a graphical representation of two typically used filters, 525/30 and 585/42, to detect these fluorophores. Marked by the arrow is the portion of the FITC spectrum that will be detected in the PE detector (585/42), which must be subtracted from the PE signal using compensation. This process becomes even more complicated when photons from multiple dyes are detected in each PMT.
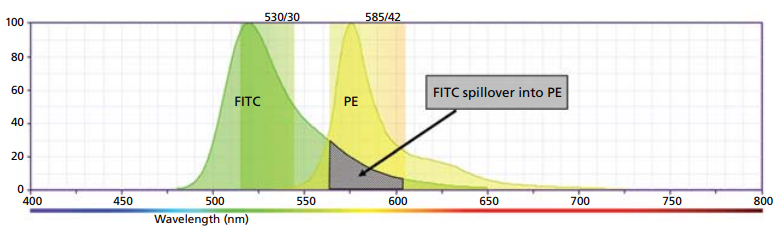 Figure 2. Example of FITC spillover into the PE channel.
Figure 2. Example of FITC spillover into the PE channel.
When a sample is stained with both fluorophores, a double positive population will be observed without compensation. However, when using software to calibrate the compensation, the true level of staining is revealed. The software calculates the overflow values and applies it to the data to obtain correct compensation data. After compensating, we can see that there are actually no double positive cells, which is to be expected from these mutually exclusive markers.
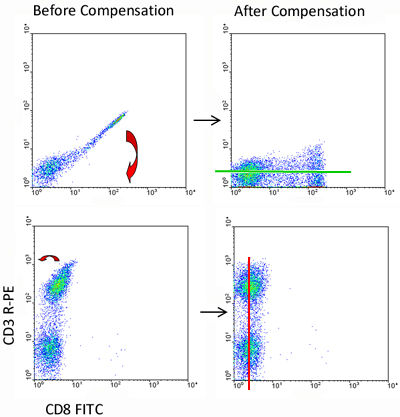 Figure 3. Fluorescence compensation corrects for spectral overlap.
Figure 3. Fluorescence compensation corrects for spectral overlap.
Compensation Controls
Since compensation control is critical to determine the positive or negative of a given marker in an experiment, there are a few basic principles to keep in mind when designing compensation control for an experiment.
- In order to exclude the double-positive cells, the compensation control should be stained with a single color only.
- Negative and positive cells must have the same level of auto-fluorescence to avoid overcompensation.
- If an alternative marker is used to set compensation, it is crucial that the marker is at least as bright as the marker in the experimental sample.
- Compensation control samples must contain the same fluorochrome as the experimental samples.
References
- Roederer M. et al.; Spectral compensation for flow cytometry: visualization artefacts, limitations, and caveats. Cytometry, 2001, 45: 194-205.
- Tung J M. et al.; New approaches to fluorescence compensation and visualization of FASC data. Clinical Immunology, 2004, 110(3): 277-283.
Cell Services:
Cell Line Testing and Assays: