Detection of Cytokines by Flow Cytometry
Intracellular cytokine detection by flow cytometry has emerged as the primary technique for studying cytokine production at the single cell level. Multi-parameter flow cytometry allows simultaneous detection of two or more cytokines within a single cell. This capability, coupled with the high throughput inherent in the instrumentation, gives intracellular cytokine staining a significant advantage over existing single-cell techniques such as ELISPOT, limiting dilution, and T cell cloning. Here, a protocol in which four-color staining for CD4, IFN-γ, IL-4, and IL-2, resulting in the enumeration of Th1 versus Th2 cells, is described. Depending on the cell population, cytokines of interest and the complexity of the cytometer, investigators can limit themselves to two parameters or use three to four parameters.
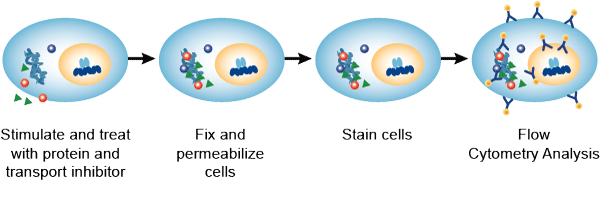
Materials
| Peripheral blood | Complete RPMI-10 medium |
| 10 mM ionomycin in DMSO (add 5 mg Ca2+ ionomycin per 0.67 mL DMSO) | 200 µg/mL phorbol myristate acetate (PMA) in 100% ethanol |
| 10 mg/mL brefeldin A (BFA) in DMSO | 4% paraformaldehyde |
| Phosphate buffered saline containing 0.1% bovine serum albumin (PBS/BSA) | Phosphate buffered saline containing 0.1% saponin (PBS-S) |
| PE/Cy5 or PerCP/Cy5.5-conjugated anti-human CD4 | Unlabeled irrelevant isotype-control MAbs matching each of the anti-cytokine Ig isotypes |
| Unlabeled and APC-labeled anti-IL-2 MAb | Unlabeled and FITC-labeled anti-IFN-γ MAb |
| Unlabeled and PE-labeled anti-IL-4 MAb | Cell scraper or disposable transfer pipets |
| 24-well tissue culture plates | Refrigerated centrifuge |
Prepare and Activate PBMC
- Peripheral blood mononuclear cells (PBMC) are prepared using the methods detailed in Cell Preparation for Flow Cytometry.
- Resuspend PBMC in complete RPMI-10 medium at a concentration of 4 x 106 cells/mL.
- Warm cells to 37°C and plate 4 x 106 cells into each well of a 24-well tissue culture plate.
- Pre-warm a sufficient quantity of complete RPMI-10 medium to 37°C. For every 5 mL of medium, add 1 µL each of 10 mM ionomycin and 200 µg/mL PMA, and 10 µL of 10 mg/mL BFA. Mix well.
Note: All of the chemicals used to activate the cells are nonpolar and will stick to plastic; therefore, once diluted, the activators must be used within 15 to 30 min of preparation. Handle all of these stock solutions with gloves. - Add 1 mL of the complete RPMI-10 medium containing PMA, ionomycin, and BFA to each well (final concentrations: 2 x 106 cells/mL, 20 ng/mL PMA, 1 µM ionomycin, and 10 µg/mL BFA) of the 24-well plate containing the cells.
Note: Controls to be used depend on the sample being analyzed. In addition, some wells containing “experimental” samples should be incubated in the absence of PMA/ionomycin. - Incubate 6 h in a 37°C, 5% CO2 incubator. This time period is optimal for IL-4 and IL-5, and is a reasonable compromise for IL-2 and IFN-γ. These kinetics are optimized for PMA/ionomycin activation; each investigator should determine the optimal conditions for different stimuli or cytokines of interest.
- Add 1 mL of cold PBS (no BSA) and vortex.
- Centrifuge 5 min at 300 g, 4°C and then aspirate supernatant.
Cell Surface Staining of PBMC
- Aspirate supernatant and break up the cell pellet by knocking the bottom of the tube.
- Prepare a solution of CD4 MAb conjugate in 200 µL of PBS/BSA at the desired final MAb concentration. Keep antibody solutions ice-cold.
- Add 200 µL of MAb solution to the cell pellet and resuspend cells by repeat pipetting. Incubate 20 min in the dark on ice.
- Add 1 mL ice-cold PBS (no BSA) to each tube and gently vortex. Centrifuge 10 min at 300 g, 4°C.
Note: It is important that all washes and incubation be done on ice to decrease secretion of intracellular cytokine.
Fixation of PBMC
- Add 500 µL of 4% paraformaldehyde (pre-warmed to 37°C) to each tube and incubate 5 min at room temperature, vortex periodically three to four times. This agitation is essential to avoid cell clumping. Add 2 mL ice-cold PBS/BSA and vortex.
- Centrifuge 5 min at 500 g, 4°C and then aspirate supernatant.
Intracellular Cytokine Staining
- Resuspend the pellet in one tube with 50 µL PBS-S containing 100 µg/mL each of unlabeled specific anti-IFN-γ, anti-IL-4, and anti-IL-2 MAbs. In the second tube, resuspend the pellet with 50 µL PBS-S containing 100 µg/mL unlabeled isotype-control MAbs matching each of the anti-cytokine Ig MAbs. Incubate 1 h at 4°C.
Note: 1) Isotype controls should match each of the anti-cytokine Ig isotypes. 2) Saponin is a reversible permeabilization agent, and must be present in all staining and washing buffers. - Add FITC-labeled anti-IFN-γ MAb, PE-labeled anti-IL-4 MAb, and APC-labeled anti-IL-2 MAb at optimal concentration to all tubes and incubate 30 min at 4°C.
Note: Do not wash out unlabeled antibody prior to the addition of labeled MAbs. - Wash cells twice and perform flow cytometric analysis.
Troubleshooting
| Problem | Possible causes | Recommended solutions |
| No signal or weak fluorescence intensity | Intracellular target not accessible | Check if target protein is intracellular. Extend permeabilization incubation time and ensure the permeabilization on ice with cold reagents. |
| Insufficient antibody present for detection | Increase amount/concentration of antibody. | |
| Antibody-conjugated fluorochrome too large | Fluorochromes for intracellular staining should have low molecular weight. | |
| Primary and secondary antibody are not compatible | Secondary antibody should be raised against the host species of the primary antibody. | |
| Target protein is absence or expressed at low level | Ensure the cells express target protein and it is present high enough to be detected. | |
| High fluorescence intensity | Antibody concentration too high | Reduce the amount of antibody added to each sample. Too much antibody will result in high non-specific binding. |
| Excess antibody trapped | Ensure adequate wash steps and may include Tween-20 or Triton X-100 in wash buffers. | |
| Low event rate | Low number of cells | Optimal cell concentration should be around 1 x 106 cells/mL. |
| Cells clumped or blocking the tube | Pipet the samples gently before staining and again before running the cytometer. | |
| High event rate | High number of cells | Dilute sample to 1 x 106 cells/mL. Adjust the flow rate if applicable. |
| Contamination | Repeat the staining procedures. |
References
- Foster B. et al.; Detection of intracellular cytokines by flow cytometry. Current Protocols in Immunology, 2007, 78: 6.24.1-6.24.21.
- Maecker H. T. et al.; Standardization of cytokine flow cytometry assays. BMC Immunol, 2005, 6: 13.
Cell Services:
Cell Line Testing and Assays: