Cell Attachment Assay
Cell attachment studies include the analysis from the formation of a molecular bond between the cell surface receptors and the complementary ligands (on the surface of ECM) to the observation of a population of cell responses through the cell behavior and changes of morphology during the attachment events. The adhesion events can be divided into single cell and cell population analysis. For the single cell study, the experiments are performed to analyze the interaction between the individual cell and the substrate, observing the morphological changes, studying the cellular migration, and measuring the traction forces. Population studies involve the analysis of attachment events for a group of cells. It is of great significance in determining the adhesion behavior of cells toward treatments or different physiological conditions, understanding the mechanism of cell adhesion, analyzing the biocompatibility of bio-materials for tissue engineering, cancer metastasis studies, and also the potential of drug treatments.
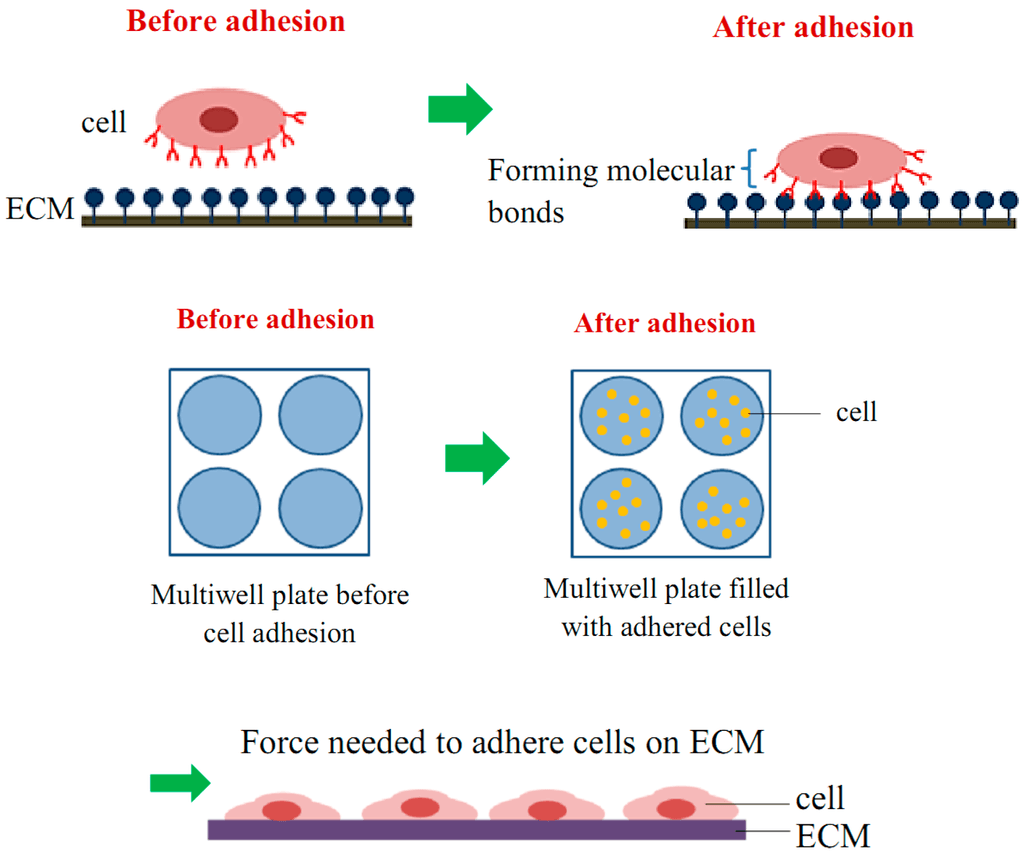 Figure 1. Schematic diagram of cell adhesion attachment events.
Figure 1. Schematic diagram of cell adhesion attachment events.
Materials
- Bovine serum albumin (BSA)
- Fetal bovine serum (FBS)
- 96-well tissue culture plate
- Dulbecco's modified eagle medium (DMEM)
- 10% (v/v) acetic acid
- 0.1% (w/v) crystal violet
- 5% (w/v) glutaraldehyde
- Phosphate buffered saline (PBS)
Attachment Assay Procedure
- Dilute adhesion molecule with PBS.
- Add the diluted adhesion molecule to the wells of the microtiter plate (100 µL/well). Leave a blank well or wells for measuring background spreading on blocked plastic.
Note: Most tissue culture plates are adequate for adhesion experiments. - Incubate at room temperature for 60 min or at 4°C overnight.
- Aspirate, add 200 µL 10 mg/mL heat-denatured BSA in divalent cation-free PBS and incubate at room temperature for 30 min.
- While the blocking is underway, prepare a suspension of the cells to be examined.
Note: It is important to guard against clumping or aggregation of cells, therefore, gentle conditions should be used when centrifuging and resuspending cells. - Count the cell density on a hemocytometer, resuspend the cells to a concentration of 5 x 105/mL for fibroblasts and 107/mL for lymphoid cells in warm DMEM medium. For experiments examining the effects of inhibitory agents, aspirate the BSA, add 50 µL of agent diluted into PBS followed by 50 µL cells. Add 50 µL PBS followed by 50 µL cells for control wells.
Note: It is our experience that the adhesion of some cell types is improved by raising the concentration of gaseous CO2 from the usual 5% to 7%. This can be particularly effective at increasing binding to poorly adhesive substrates. - The incubation time chosen for attachment assay depends on the cell type, as some cells adhere more quickly than others, but 15-20 min is usually adequate.
- Fix cells in the wells to be used for determining 100% attachment value by addition of 100 µL 5% (w/v) glutaraldehyde for 20 min at room temperature (or at 4°C overnight if necessary). Assays are usually performed in triplicate or quadruplicate.
- Remove non-adherent and loosely attached cells by either tapping the plate or gently washing the wells with PBS.
Note: 1) This is the most critical stage in an attachment assay, and needs to be optimized for each cell type used. Different cells respond differently to tapping and washing, and we recommend varying the number of tapping or washing cycles to obtain the best signal: noise ratio. 2) Attachment assay relies much more on the accuracy of pipetting, and therefore it is advisable to use a P200 pipettor rather than a multi-channel pipettor wherever possible. - Wash wells with 3 x 100 µL water.
- Stain with 100 µL 0.1% (w/v) crystal violet, 200 mM MES, pH 6.0 for 60 min at room temperature.
Note: Avoid getting crystal violet solution on the rim of the wells, as this dyes can be difficult to remove by washing. - Wash wells with 3 x 400 µL water.
- Solubilize dye in 100 µL 10% (v/v) acetic acid and incubate on orbital shaker at 150 rpm for 5 min at room temperature.
- Measure absorbance at 570 nm using a plate reader.
References
- Amelia A K. et al.; A review of cell adhesion studies for biomedical and biological applications. International Journal of Molecular Science, 2015, 16: 18149-18184.
- Humphries M J. Cell adhesion assays. Methods Mol Biol, 2009, 522: 203-210.
Cell Services:
Cell Line Testing and Assays