Clonogenic Assay
Clonogenic assay enables an assessment of the differences in reproductive viability between untreated control cells and cells that have undergone various treatments such as ionizing radiation and chemical compounds (e.g. cytotoxic agents). The assay has become the most widely accepted technique in radiation biology and has been widely used for evaluating the radiation sensitivity in different cell lines. In addition, the clonogenic assay is commonly used for monitoring the effectiveness of radiation-modified compounds and for determining the effects of cytotoxic drugs and other anti-cancer therapeutics on colony forming ability.
Clonogenic assay is a cell biology technique for studying the efficacy of specific agents on cell survival and proliferation. It is frequently used in cancer research to determine the effect of drugs or radiation on proliferating tumor cells. Any types of cells can be used, but since the goal of these experiments in oncological research is to find more effective cancer treatments, human tumor cells are a typical choice.
Key Features
- Clonogenic assay is the gold standard for assessing cell reproduction or proliferation in radiobiology.
- Analysis of the data provides important information on cell radiosensitivity.
- Useful tool for intracellular and intercellular radiation comparisons.
Materials
Cell culture medium
Trypsin-EDTA
Phosphate buffered saline (PBS)
0.5% crystal violet
Fixation solution (acetic acid/methanol 1:7)
Incubator
Hemocytometer
Stereomicroscope
Cell culture dishes or plates
Cell Preparation
1) Remove the medium above the cells.
2) Wash the cells with PBS.
3) Trypsinize cells to produce a single cell suspension. The trypsin solution should remain on the cells until they round up, which may be observed under the microscope.
4) Adding sufficient volume of medium supplemented with serum to neutralize the trypsin solution. Detach the cells by pipetting up and down the medium with cells.
5) Count the cells using a hemocytometer. The accurate number of cells are required to obtain the correct data for plating efficiency (PE) or survival fraction (SF).
6) Dilute the cell suspension into the desired seeding concentration and seed into dishes or plates as desired. The dilutions have to be performed accurately to seed the correct number of cells.
Clonogenic Assay Setup
There are two different ways to perform studies using this assay. (A) Cells are plated before treatment. This method is often used to rapidly screen the sensitivity of cells to different treatments. (B) Cells are treated in dishes and subsequently re-plated in appropriate dilutions to assess clonogenic ability. This method is particularly useful in radiobiological research to determine potentially lethal or sub-lethal damage repair.
- (A) Plating before treatment
1) Collect cells and re-plate the appropriate number of cells per dish or per well. The number of cells depend on the severity of the treatment, if you do not know the appropriate effect, use different dilutions of different cell numbers.
2) Incubate the cells for a few hours in a CO2 incubator at 37°C and allow them to attach to the dish or plate.
3) Treat the cells as needed with chemicals, radiation or a combination of both.
4) Incubate the cells in a CO2 incubator at 37°C for 1-3 weeks until the control dishes form sufficiently large colonies (50 cells per colony is the minimum for counting).
- (B) Plating after treatment
1) Collect cells after treatment. 100 or up to 104 cells can be pipetted into the test wells. It is always better to keep the cells on ice before re-plating. If you do not know the severity of the treatments, prepare serial dilutions with different numbers of cells.
2) Incubate the cells in a CO2 incubator at 37°C for 1-3 weeks until the control dishes form sufficiently large colonies (50 cells per colony is the minimum for counting).
Fixation and Staining
1) Remove the medium and rinse carefully with 10 mL PBS.
2) Remove the PBS and add 2-3 mL of fixation solution.
3) Incubate at room temperature for 5 min.
4) Remove the fixation solution and add 0.5% crystal violet.
5) Incubate at room temperature for 2 hours.
6) Remove the crystal violet carefully and rinse with tap water. Instead of placing the dishes or plates under the running tap, fill the sink with water and immerse the dishes or plates carefully.
7) Air-dry the dishes or plates at room temperature.
Counting the Colonies
1) Count the number of colonies using a stereomicroscope or an automatic counting "colony counter".
2) Calculate the plating efficiency (PE) and surviving fraction (SF).

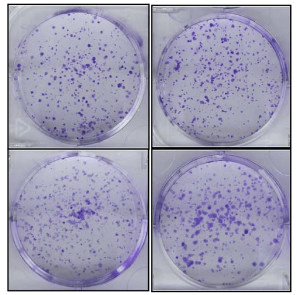 Figure 1. Colony formation assay of PC3 cells.
Figure 1. Colony formation assay of PC3 cells.
References
- Fedr R, et al.; Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytometry A, 2013, 83(5): 472-482.
- Stanley Borowicz, et al.; The soft ager colony formation assay. J Vis Exp, 2014, 92: 51998.
- Haloom Rafehi, et al.; Clonogenic assay: adherent cells. J Vis Exp, 2011, 49: 2573.
Cell Services:
Cell Line Testing and Assays: