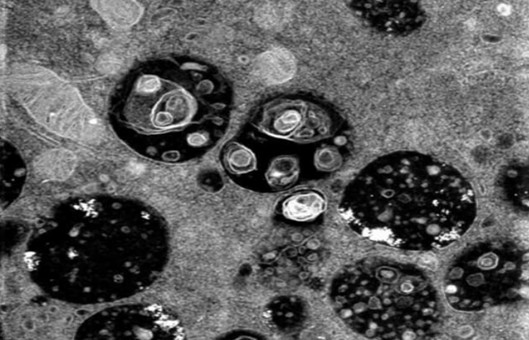
Transmission Electron Microscopy (TEM) Protocol
GUIDELINE
Transmission electron microscopy is a technique for observing the fine details of organelles in cells or tissues. This protocol is to be used to examine the membrane structure in cells with or without virus infection. Modifications should be made if users want to get images from tissues.
METHODS
- After treatment, collecting cells (1 x 106) with 0.5% trypsin-EDTA and washing cells with 0.5 ml 0.1 M phosphate buffer (PB) in an Eppendorf tube by centrifuge (300xg, 5 min).
- Add 0.5 ml paraformaldehyde (4% in PB) to fix cells at room temperature (RT) for at least 30 min (or store cells at 4°C for long-term storage).
- Wash cells 3 times with 0.5 ml 0.1 M PB by centrifuge (300xg, 5 min).
- Add 0.5 ml Osmium tetraoxide (1% in PB) into the tube and incubate cells for 1 h at RT.
- Wash cells 3 times with 0.5 ml 0.1 M PB by centrifuge (300xg, 5 min).
- Dehydrate cells by adding 0.5 ml 70% alcohol for 5 min twice. Centrifuge (300xg, 5 min), remove alcohol.
- Dehydrate cells by adding 0.5 ml 85% alcohol for 5 min twice. centrifuge (300xg, 5 min), remove alcohol.
- Dehydrate cells by adding 0.5 ml 95% alcohol for 5 min three times. Centrifuge (300xg, 5 min), remove alcohol.
- Dehydrate cells by adding 0.5 ml 100% alcohol for 10 min five times. Centrifuge (300xg, 5 min), remove alcohol.
- Add 0.5 ml propylene oxide into tubes and incubate for 3 min twice at RT. Centrifuge (300xg, 5 min), remove propylene oxide.
- Add 1 ml (propylene oxide: Epon = 1:1) into tubes and incubate for 1 h at RT. Centrifuge (300xg, 5 min), remove propylene oxide: Epon.
- Add 1 ml pure Epon into tubes and put the samples into the vacuum drying oven to vacuum for 1 h at RT. Remove Epon.
- Add 1 ml pure Epon into tubes and vacuum for 1 h at RT. Remove Epon.
- Add 1 ml pure Epon into tubes and vacuum overnight at RT.
- Put tubes into 60°C oven for at least 48 h.
- Trimming-remove the edge of the block.
- Get a thick section (1 μm) with Ultracut using a diamond knife, put the thick section on a slide, and stain with 0.5% toluidine blue for around 40 s to 1 min (until the edge is dried), wash for 1 min with water, and observe under microscopy to find the cells.
- Once we found the cells on the thick section, change the thickness of Ultracut to get thin sections (65-70 nm).
- Put the thin sections on the grids (3-4 sections on one grid).
- Stain sections with uranyl acetate and lead citrate for 3 min sequentially.
- Wash the grids with water for 1 minute and let them dry completely.
- Get images from a digital camera on TEM with identical magnificence.
Creative Bioarray Relevant Recommendations
- Creative Bioarray has scientists and imaging laboratories to perform the full comprehensive Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM) Services for the biological sciences and clinical research.
| Assays Available | Description |
| Particle Size Analysis | Creative Bioarray can analyze the size of particles and nanoparticles down to 1 nm in size. We use SEM or TEM. |
| Negative Staining-TEM for Samples | Isolated organelles, bacteria, and viruses pose a problem when imaging in a TEM since they do not have high enough contrast (they are not electron-dense). For this purpose, Creative Bioarray uses negative staining. |
| Cryogenic Transmission Electron Microscopy (cryo-TEM) | Cryo-TEM has evolved into an essential tool for the characterization of colloidal drug delivery systems. |
NOTES
Put all droppers and syringes into the 60°C oven the day before this procedure to remove all the water inside all the stuff.