Toxicokinetic Characterization of Hexamethylenetetramine
Toxics. 2023 Mar 31; 11 (4): 337.
Authors: Kim W, Kim E, Lee J, Song CH, Jung W, Shin S, Kim KB, Shin BS, Kim TH.
INTRODUCTION
Hexamethylenetetramine, an aldehyde-releasing agent, is used as a preservative in various food, cosmetics, and medical treatments, such as a treatment for urinary tract infections. It has been reported to be allergenic in contact with the skin, with the additional possibility of causing toxicity once absorbed systemically. Despite its potential toxicity, there are no reports on the in vivo bioavailability of hexamethylenetetramine following oral or dermal administration.
METHODS
- Animals. Male Sprague-Dawley rats (7-8 weeks old, body weight 225-250 g) were kept in plastic cages with free access to a standard diet. For serial blood sampling, rats were anesthetized by intra-peritoneal injection, and polyethylene (PE) tubing was intubated into the jugular vein.
- IV Injection Study. Hexamethylenetetramine was dissolved in saline for iv injection at doses of 0.2 and 0.5 mg/kg. Blood samples (0.2 mL each) were collected via jugular vein at 0, 2, 5, 10, 15, and 30 min, and 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after injection.
- Oral Administration Study. Hexamethylenetetramine was dissolved in saline for iv injection at doses of 0.2 and 0.5 mg/kg. After overnight fasting, a dosing solution was injected via the penile vein. The injected dosing volume was constantly 1 mL/kg regardless of the dose. Blood samples (0.2 mL each) were collected via jugular vein at 0, 2, 5, 10, 15, and 30 min, and 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after injection.
- Percutaneous Absorption Study. For the percutaneous absorption study, gel formulation consisting of hexamethylenetetramine (0.15 or 0.6%), carbomer 940 (1.1%), and distilled water (98.75 or 98.3%) was used. After overnight fasting, 5 mg/cm2 of hexamethylenetetramine gel was evenly applied to 4.5×4.5 cm2 of the shaved area. Blood samples (0.2 mL each) were collected via jugular vein at 0, 5, 15, and 30 min, and 1, 1.5, 2, 4, 6, 8, 10, 12, and 24 h after dosing.
- Browse our recommendations
As an established leader in the field of cell science, Creative Bioarray offers a range of high-quality products and services for our customers' research, including but not limited to the products in the table below.
| Product/Service Types | Description |
| Administration Routes and Biofluid Sampling | A variety of administration routes and collection of biological samples are offered, as well as comprehensive bioanalytical package services. |
| TK testing services | Toxicokinetic (TK) studies assess the relationship between the toxic dose in vivo and its toxic effects. |
| In Vivo Toxicity Study | Our pre-clinical toxicity assays include administration routes ranging from acute to chronic, from single-dose administration to multiple-dose administration. Moreover, various drug administration routes and animal models are provided. |
RESULTS
- Average plasma concentration-time profiles of hexamethylenetetramine obtained after iv injection, oral administration, and percutaneous administration are shown in the following figure.
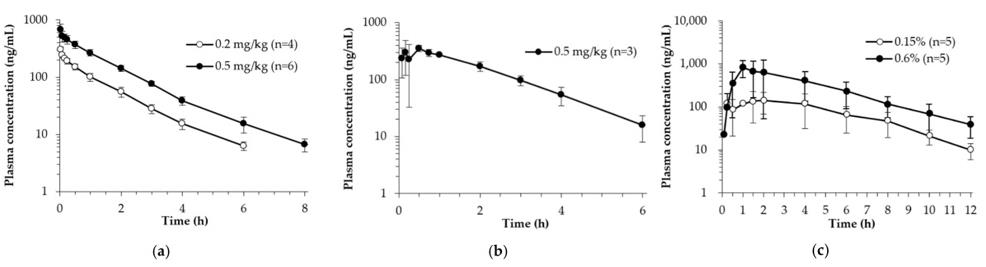 Fig. 1 Average plasma concentration-time profiles of hexamethylenetetramine obtained after (a) iv injection, (b) oral, and (c) percutaneous administration in rats.
Fig. 1 Average plasma concentration-time profiles of hexamethylenetetramine obtained after (a) iv injection, (b) oral, and (c) percutaneous administration in rats.
- After iv injection of 0.2 and 0.4 mg/kg of hexamethylenetetramine, a mono-exponential decline was observed in the plasma concentrations. Following oral administration, hexamethylenetetramine was quantifiable in the first plasma samples (5 min) and reached Cmax at about 0.5 h. In the percutaneously administered group, hexamethylenetetramine was relatively slowly absorbed. The Cmax was reached approximately 3 h after dosing.
- Following iv injection, t1/2 was estimated as 1.26-1.36 h, and plasma concentration was below that of the LLOQ (BLOQ) at 8 h. For the oral administration, the t1/2 was estimated as 1.12 h, and absolute bioavailability was calculated as 89.83%. Following percutaneous administration, t1/2 was estimated as 1.69-2.43 h.
SUMMARY
Most of the orally and percutaneously administered hexamethylenetetramine was absorbed into the systemic circulation. Following iv injection, the plasma concentration of hexamethylenetetramine showed mono-exponential decay, with an elimination half-life of about 1.3 h. Following oral administration, the Tmax reached an average of 0.47 h, and bioavailability was estimated at 89.93%. After percutaneous administration, it reached Cmax on average at 2.9-3.6 h.
RELATED PRODUCTS & SERVICES
Reference
- Kim W, et al. (2023). "Development of an LC-MS/MS Assay and Toxicokinetic Characterization of Hexamethylenetetramine in Rats." Toxics. 11 (4), 337.