Report on Generation of iPSC Lines from PBMCs
Stem Cell Research. 2023 Jun; 69: 103088.
Authors: Gao Y, Cheng Y, Wen J, Xiao D, Xu J, Cai C, Hu J.
INTRODUCTION
The source cell types for reprogramming include skin fibroblasts, peripheral blood mononuclear cells (PBMC), and epithelial cells. Although fibroblasts from skin or other sources were originally used in many studies to generate IPSCs, mononuclear cells from peripheral blood have been widely recognized as a more convenient and nearly unlimited resource for cellular reprogramming. Here, we provide the use of non-integrated, plasmid-based reprogramming to generate and expand hiPSC from human PBMC. The resulting reprogrammed hiPSC cells can rapidly expand and differentiate.
METHODS
- PBMCs were isolated by centrifugation at 1200×g for 15 min, and cells in the middle white layer were collected and transferred to a new tube. Cells were washed by DPBS twice and centrifuged at 300 g for 10 min at each time.
- PBMCs can be rapidly expanded when treated with the appropriate media containing PBMC stimulating factors. PBMCs were grown for up to 4 days to reach a sufficient amount for reprogramming. The cells were electroporated with four episomal plasmids The cells were electroporated with four episomal plasmids. The medium was changed every day until iPSC colonies were manually picked up. iPSC colonies were mechanically isolated and further expanded in culture.
- Cells were fixed with 4% Paraformaldehyde for 15 min and then washed three times with PBS. After being treated with perforation and blocking buffer for 30 min at room temperature, cells were incubated with primary antibodies of pluripotency markers overnight at 4°C. On the next day, cells were incubated with secondary antibodies at room temperature for 2 h. Subsequently, after stained nuclei with DAPI, cells were observed and photographed with a fluorescence microscope.
- iPSCs were harvested, fixed, filtered, and incubated with directly conjugated antibodies for 40 min at 4°C. Samples were measured on a flow cytometer. The karyotype and STR were evaluated of all iPSC lines after passage 15 generation.
- iPSCs were expanded and resuspended with 100 μL Matrigel and then injected into the underarm subcutaneous of NOD-SCID mice with a cell number of 2.5 million for each mouse. All the teratomas were formed within 8 weeks and collected for paraffin section and hematoxylin and eosin staining after the mice were sacrificed. The differentiation potential of iPSCs in vivo was evaluated according to the histological analysis results.
- Browse our recommendations
| Product/Service Types | Description |
| Human iPSC Line from PBMCs | Creative Bioarray offers the human iPSC line that was derived from human peripheral blood mononuclear cells (PBMCs) by ectopic expression of OCT4, SOX2, KLF4, and L-MYC genes using episomal plasmids. |
| Karyotyping (G-Banded) Service | Creative Bioarray has performed many karyotyping (banded chromosome analysis) services for cell line authentication and some specific research. In providing karyotyping information, we can promote the advancement of biology projects. |
| Teratoma Formation Assay | Creative Bioarray has established a standard protocol for teratoma assay based on subcutaneous co-transplantation of human iPSCs with mitotically inactivated feeder cells and Matrigel into immunodeficient mice. |
| DNA/RNA Extraction Service | Creative Bioarray offers extraction services of DNA and RNA including large mRNA, microRNA, and siRNA from a wide range of starting materials with different scales/volumes for most applications or bespoke solutions. |
RESULTS
- Reprogramming factors were overexpressed into the expanded PBMCs and after 24 days of culture, we isolated the colonies morphologically similar to pluripotent stem cells. The monoclonal culture of reprogramming cells for purification and morphology. We generated two iPS cell lines for each donor and named SZBKi001-A, SZBKi001-B, SZBKi002-A, and SZBKi002-B. Compared with the PBMCs, reprogramming cells showed a significantly higher gene expression of the pluripotent markers similar to a normal hESC H9 (RT-PCR). The flow cytometry assay demonstrated that SOX2, OCT4, and NANOG were highly expressed in reprogramming cells. Furthermore, the immunofluorescence assay exhibited a consistent result with the above data which indicated that the PBMCs we obtained from the donors were successfully reprogrammed into iPSCs. Meanwhile, the non-integration of episomal plasmids was determined by genomic PCR.
- Karyotype analysis showed that all the iPS cell lines had normal male karyotypes with the correct number (46, XY). Short tandem repeat analysis confirmed that the four iPS cell lines shared the same information with their corresponding PBMCs (available upon request). iPS cell differentiation potential was tested by a commercially available trilineage differentiation kit and was tested by flow cytometry. Specific markers in each germ layer displayed obvious expressions which proved our iPSCs could differentiate into other cells in all germ layers. In addition, we also detected other characteristic markers in each germ layer by RT-PCR and gave stronger evidence that the iPSCs we generated had trilineage differentiation ability. Representative tissues shown in the teratoma assay confirmed that the reprogramming iPSCs could differentiate into the cells in all three germ layers in vivo.
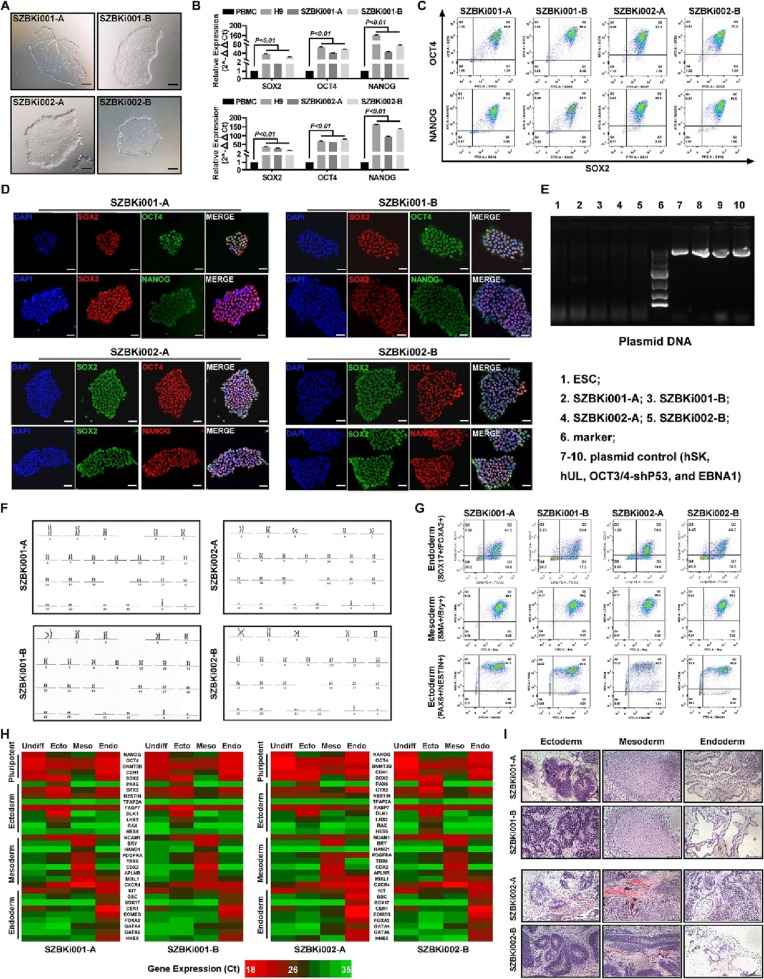 Fig. 1 Characterization and validation of iPSC lines derived from PBMCs.
Fig. 1 Characterization and validation of iPSC lines derived from PBMCs.
SUMMARY
We reprogrammed the PBMCs by using non-integrative non-viral vectors liposome electro-transfer containing the reprogramming factors OCT4, SOX2, KLF4, and c-MYC. The iPSC lines exhibited a normal karyotype with their corresponding PBMCs and exhibited significant cellular pluripotency. Teratoma formation assay revealed that the iPSCs we generated could differentiate into three embryonic germ layers. Our study provides a more effective procedure for peripheral blood monocyte reprogramming to iPSC and promotes its future application.
RELATED PRODUCTS & SERVICES
REFERENCE
- Gao Y, et al. (2023). "Efficient generation of induced pluripotent stem cell lines from peripheral blood mononuclear cells." Stem Cell Res. 69: 103088.